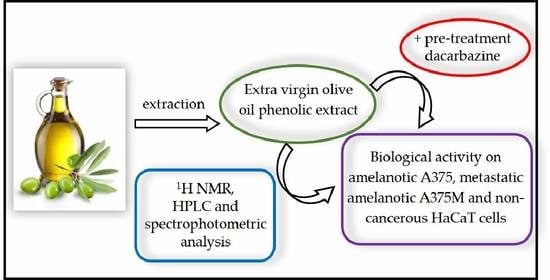
Extra Virgin Olive Oil Secoiridoids Modulate the Metabolic Activity of Dacarbazine Pre-Treated and Treatment-Naive Melanoma Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. EVOO’s Polyphenolic Profile
2.1.1. Total Polyphenols, o-Diphenols, and Total Flavonoids
2.1.2. Secoiridoids Determined by NMR Spectroscopy
2.1.3. Phenolic Compounds Determined by HPLC Chromatography
2.2. Biological Effects of EVOO-PEs, DTIC, OCEIN, and OCAL on the Metabolic Activity of A375, A375M, and HaCaT Cells
2.3. Post-Treatment Biological Effects of EVOO-PEs on the Metabolic Activity after Pre-Incubation with DTIC
3. Materials and Methods
3.1. Reagents and Standards
3.2. Olive Oil Samples
3.3. Extraction of Polyphenolic Compounds for Spectrophotometric and HPLC-DAD Analysis
3.4. Polyphenolic Compounds Determined by Spectrophotometric Analysis
3.4.1. Total Polyphenols Analysis
3.4.2. o-Diphenols Analysis
3.4.3. Total Flavonoids Analysis
3.5. Secoiridoids Determined by NMR Spectroscopy
3.5.1. Extraction of Phenolic Compounds/Secoiridoids for 1H NMR Analysis
3.5.2. 1H qNMR Analysis
3.5.3. 1H NMR Calibration Curves and Quantitative Determination
3.6. Phenolic Compounds Determined by HPLC Chromatography
HPLC-DAD Analysis
3.7. Cell Culture and Biological Activity
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea Europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean Diet and Health Status: Meta-Analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Servili, M.; Esposto, S.; Fabiani, R.; Urbani, S.; Taticchi, A.; Mariucci, F.; Selvaggini, R.; Montedoro, G.F. Phenolic Compounds in Olive Oil: Antioxidant, Health and Organoleptic Activities According to Their Chemical Structure. Inflammopharmacology 2009, 17, 76–84. [Google Scholar] [CrossRef]
- Riera, R.; Bagattini, A.M.; Pacheco, R.L.; Pachito, D.V.; Roitberg, F.; Ilbawi, A. Delays and Disruptions in Cancer Health Care Due to COVID-19 Pandemic: Systematic Review. JCO Glob. Oncol. 2021, 7, 311–323. [Google Scholar] [CrossRef]
- Marson, J.W.; Maner, B.S.; Harding, T.P.; Meisenheimer, J.; Solomon, J.A.; Leavitt, M.; Levin, N.J.; Dellavalle, R.; Brooks, I.; Rigel, D.S. The magnitude of COVID-19’s effect on the timely management of melanoma and nonmelanoma skin cancers. J. Am. Acad. Dermatol. 2021, 84, 1100–1103. [Google Scholar] [CrossRef]
- Cancer Burden Statistics and Trends Across Europe ECIS. Available online: https://ecis.jrc.ec.europa.eu (accessed on 27 April 2022).
- Hrvatski Zavod za Javno Zdravstvo, Registar za Rak Republike Hrvatske. Incidencija Raka u Hrvatskoj 2019. Bilten 44, Zagreb. 2021. Available online: https://www.hzjz.hr/wp-content/uploads/2021/12/Bilten44_2019.pdf (accessed on 27 April 2022).
- Lev, D.C.; Onn, A.; Melinkova, V.O.; Miller, C.; Stone, V.; Ruiz, M.; McGary, E.C.; Ananthaswamy, H.N.; Price, J.E.; Bar-Eli, M. Exposure of Melanoma Cells to Dacarbazine Results in Enhanced Tumor Growth and Metastasis In Vivo. J. Clin. Oncol. 2004, 22, 2092–2100. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Kirkwood, J.M. Re-Evaluating the Role of Dacarbazine in Metastatic Melanoma: What Have We Learned in 30 Years? Eur. J. Cancer 2004, 40, 1825–1836. [Google Scholar] [CrossRef]
- Luther, C.; Swami, U.; Zhang, J.; Milhem, M.; Zakharia, Y. Advanced Stage Melanoma Therapies: Detailing the Present and Exploring the Future. Crit. Rev. Oncol. Hematol. 2019, 133, 99–111. [Google Scholar] [CrossRef]
- Mirzaei, H.; Naseri, G.; Rezaee, R.; Mohammadi, M.; Banikazemi, Z.; Mirzaei, H.R.; Salehi, H.; Peyvandi, M.; Pawelek, J.M.; Sahebkar, A. Curcumin: A New Candidate for Melanoma Therapy? Int. J. Cancer 2016, 139, 1683–1695. [Google Scholar] [CrossRef]
- Prasanth, M.; Sivamaruthi, B.; Chaiyasut, C.; Tencomnao, T. A Review of the Role of Green Tea (Camellia Sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niedzwiecki, A.; Roomi, M.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sajadimajd, S.; Bahramsoltani, R.; Iranpanah, A.; Kumar Patra, J.; Das, G.; Gouda, S.; Rahimi, R.; Rezaeiamiri, E.; Cao, H.; Giampieri, F.; et al. Advances on Natural Polyphenols as Anticancer Agents for Skin Cancer. Pharmacol. Res. 2020, 151, 104584. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Czerwińska, M.; Kiss, A.K.; Naruszewicz, M. A Comparison of Antioxidant Activities of Oleuropein and Its Dialdehydic Derivative from Olive Oil, Oleacein. Food Chem. 2012, 131, 940–947. [Google Scholar] [CrossRef]
- Nguyen, T.; Sherratt, P.J.; Pickett, C.B. Regulatory Mechanisms Controlling Gene Expression Mediated by the Antioxidant Response Element. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 233–260. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as Potential Antioxidant Therapeutic Agents: Mechanism and Actions. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Aziz, M.A.; Sarwar, M.S.; Akter, T.; Uddin, M.S.; Xun, S.; Zhu, Y.; Islam, M.S.; Hongjie, Z. Polyphenolic Molecules Targeting STAT3 Pathway for the Treatment of Cancer. Life Sci. 2021, 268, 118999. [Google Scholar] [CrossRef]
- Weng, C.-J.; Yen, G.-C. Chemopreventive Effects of Dietary Phytochemicals against Cancer Invasion and Metastasis: Phenolic Acids, Monophenol, Polyphenol, and Their Derivatives. Cancer Treat. Rev. 2012, 38, 76–87. [Google Scholar] [CrossRef]
- Carpi, S.; Polini, B.; Manera, C.; Digiacomo, M.; Salsano, J.E.; Macchia, M.; Scoditti, E.; Nieri, P. MiRNA Modulation and Antitumor Activity by the Extra-Virgin Olive Oil Polyphenol Oleacein in Human Melanoma Cells. Front. Pharmacol. 2020, 11, 574317. [Google Scholar] [CrossRef]
- De Carvalho, A.L.M.B.; Caselli, F.; Rodrigues, V.; Paiva-Martins, F.; Marques, M.P.M. Antiproliferative Activity of Olive Oil Phenolics against Human Melanoma Cells. Lett. Drug Des. Discov. 2017, 14, 1053–1059. [Google Scholar] [CrossRef]
- Fogli, S.; Arena, C.; Carpi, S.; Polini, B.; Bertini, S.; Digiacomo, M.; Gado, F.; Saba, A.; Saccomanni, G.; Breschi, M.C.; et al. Cytotoxic Activity of Oleocanthal Isolated from Virgin Olive Oil on Human Melanoma Cells. Nutr. Cancer 2016, 68, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wang, J.; Peng, L. (-)-Oleocanthal Exerts Anti-Melanoma Activities and Inhibits STAT3 Signaling Pathway. Oncol. Rep. 2017, 37, 483–491. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Ingrosso, D.; Migliardi, V.; Sorrentino, A.; Donnarumma, G.; Baroni, A.; Masella, L.; Antonietta Tufano, M.; Zappia, M.; Galletti, P. Hydroxytyrosol, a Natural Antioxidant from Olive Oil, Prevents Protein Damage Induced by Long-Wave Ultraviolet Radiation in Melanoma Cells. Free Radic. Biol. Med. 2005, 38, 908–919. [Google Scholar] [CrossRef]
- Costantini, F.; Di Sano, C.; Barbieri, G. The Hydroxytyrosol Induces the Death for Apoptosis of Human Melanoma Cells. Int. J. Mol. Sci. 2020, 21, 8074. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.; Tomás, A.; Silva, S.; Bronze, M.R.; Serra, A.T.; Pojo, M. The Impact of Olive Oil Compounds on the Metabolic Reprogramming of Cutaneous Melanoma Cell Models. Molecules 2021, 26, 289. [Google Scholar] [CrossRef]
- Li, W.; Sperry, J.B.; Crowe, A.; Trojanowski, J.Q.; Smith, A.B., III; Lee, V.M.-Y. Inhibition of Tau Fibrillization by Oleocanthal via Reaction with the Amino Groups of Tau: Oleocanthal Inhibits Tau Fibrillization. J. Neurochem. 2009, 110, 1339–1351. [Google Scholar] [CrossRef] [Green Version]
- Pang, K.-L.; Chin, K.-Y. The Biological Activities of Oleocanthal from a Molecular Perspective. Nutrients 2018, 10, 570. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, D.E.; Hurst, R.D. Polyphenolic Phytochemicals Just Antioxidants or Much More? Cell. Mol. Life Sci. 2007, 64, 2900–2916. [Google Scholar] [CrossRef]
- Montedoro, G.; Servili, M.; Baldioli, M.; Miniati, E. Simple and Hydrolyzable Phenolic Compounds in Virgin Olive Oil. 1. Their Extraction, Separation, and Quantitative and Semiquantitative Evaluation by HPLC. J. Agric. Food Chem. 1992, 40, 1571–1576. [Google Scholar] [CrossRef]
- Sánchez de Medina, V.; Miho, H.; Melliou, E.; Magiatis, P.; Priego-Capote, F.; Luque de Castro, M.D. Quantitative Method for Determination of Oleocanthal and Oleacein in Virgin Olive Oils by Liquid Chromatography–Tandem Mass Spectrometry. Talanta 2017, 162, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Vulcano, I.; Halabalaki, M.; Skaltsounis, L.; Ganzera, M. Quantitative Analysis of Pungent and Anti-Inflammatory Phenolic Compounds in Olive Oil by Capillary Electrophoresis. Food Chem. 2015, 169, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, R.; Addeo, F.; Paolillo, L. 1H And 13C NMR of Virgin Olive Oil. An Overview. Magn. Reson. Chem. 1997, 35, S133–S145. [Google Scholar] [CrossRef]
- Rotondo, A.; Mannina, L.; Salvo, A. Multiple Assignment Recovered Analysis (MARA) NMR for a Direct Food Labeling: The Case Study of Olive Oils. Food Anal. Methods 2019, 12, 1238–1245. [Google Scholar] [CrossRef]
- Del Cano-Ochoa, S.; Ruiz-Aracama, A.; Guillen, M.D. Potential of Nuclear Magnetic Resonance for a Discriminant Characterization of PDO VOOs. Eur. J. Lipid Sci. Technol. 2019, 121, 1800137. [Google Scholar] [CrossRef]
- Olmo-Cunillera, A.; López-Yerena, A.; Lozano-Castellón, J.; Tresserra-Rimbau, A.; Vallverdú-Queralt, A.; Pérez, M. NMR Spectroscopy: A Powerful Tool for the Analysis of Polyphenols in Extra Virgin Olive Oil. J. Sci. Food Agric. 2020, 100, 1842–1851. [Google Scholar] [CrossRef]
- Klikarova, J.; Rotondo, A.; Cacciola, F.; Ceslova, L.; Dugo, P.; Mondello, L.; Rigano, F. The Phenolic Fraction of Italian Extra Virgin Olive Oils: Elucidation Through Combined Liquid Chromatography and NMR Approaches. Food Anal. Methods 2019, 12, 1759–1770. [Google Scholar] [CrossRef]
- Ruiz-Aracama, A.; Goicoechea, E.; Guillén, M.D. Direct Study of Minor Extra-Virgin Olive Oil Components without Any Sample Modification. 1H NMR Multisupression Experiment: A Powerful Tool. Food Chem. 2017, 228, 301–314. [Google Scholar] [CrossRef]
- Karkoula, E.; Skantzari, A.; Melliou, E.; Magiatis, P. Direct Measurement of Oleocanthal and Oleacein Levels in Olive Oil by Quantitative 1H NMR. Establishment of a New Index for the Characterization of Extra Virgin Olive Oils. J. Agric. Food Chem. 2012, 60, 11696–11703. [Google Scholar] [CrossRef]
- Diamantakos, P.; Giannara, T.; Skarkou, M.; Melliou, E.; Magiatis, P. Influence of Harvest Time and Malaxation Conditions on the Concentration of Individual Phenols in Extra Virgin Olive Oil Related to Its Healthy Properties. Molecules 2020, 25, 2449. [Google Scholar] [CrossRef]
- Siddique, A.B.; Ebrahim, H.; Mohyeldin, M.; Qusa, M.; Batarseh, Y.; Fayyad, A.; Tajmim, A.; Nazzal, S.; Kaddoumi, A.; El Sayed, K. Novel Liquid-Liquid Extraction and Self-Emulsion Methods for Simplified Isolation of Extra-Virgin Olive Oil Phenolics with Emphasis on (-)-Oleocanthal and Its Oral Anti-Breast Cancer Activity. PLoS ONE 2019, 14, e0214798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goren, L.; Zhang, G.; Kaushik, S.; Breslin, P.A.S.; Du, Y.-C.N.; Foster, D.A. (-)-Oleocanthal and (-)-Oleocanthal-Rich Olive Oils Induce Lysosomal Membrane Permeabilization in Cancer Cells. PLoS ONE 2019, 14, e0216024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosillo, M.A.; Alarcon-de-la-Lastra, C.; Castejon, M.L.; Montoya, T.; Cejudo-Guillen, M.; Sanchez-Hidalgo, M. Polyphenolic Extract from Extra Virgin Olive Oil Inhibits the Inflammatory Response in IL-1 Beta-Activated Synovial Fibroblasts. Br. J. Nutr. 2019, 121, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Castellon, J.; Lopez-Yerena, A.; de Alvarenga, J.F.R.; del Castillo-Alba, J.R.; Vallverdu-Queralt, A.; Escribano-Ferrer, E.; Lamuela-Raventos, R.M. Health-promoting properties of oleocanthal and oleacein: Two secoiridoides from extra-virgin olive oil. Crit. Rev. Food Sci. Nutr. 2020, 60, 2532–2548. [Google Scholar] [CrossRef] [PubMed]
- Emma, M.R.; Augello, G.; Stefano, V.D.; Azzolina, A.; Giannitrapani, L.; Montalto, G.; Cervello, M.; Cusimano, A. Potential Uses of Olive Oil Secoiridoids for the Prevention and Treatment of Cancer: A Narrative Review of Preclinical Studies. Int. J. Mol. Sci. 2021, 22, 1234. [Google Scholar] [CrossRef] [PubMed]
- Bilušić, T.; Žanetić, M.; Ljubenkov, I.; Generalić Mekinić, I.; Štambuk, S.; Bojović, V.; Soldo, B.; Magiatis, P. Molecular Characterization of Dalmatian Cultivars and the Influence of the Olive Fruit Harvest Period on Chemical Profile, Sensory Characteristics and Oil Oxidative Stability. Eur. Food Res. Tech. 2018, 244, 281–289. [Google Scholar] [CrossRef]
- Kulišić Bilušić, T.; Melliou, E.; Giacometti, J.; Čaušević, A.; Čorbo, S.; Landeka, M.; Magiatis, P. Phenolics, Fatty Acids, and Biological Potential of Selected Croatian EVOOs: Characterization of Selected Croatian EVOOs. Eur. J. Lipid Sci. Tech. 2017, 119, 1700108. [Google Scholar] [CrossRef]
- Gutfinger, T. Polyphenols in Olive Oils. J. Am. Oil Chem. Soc. 1981, 58, 966–968. [Google Scholar] [CrossRef]
- Mateos, R.; Espartero, J.L.; Trujillo, M.; Ríos, J.J.; León-Camacho, M.; Alcudia, F.; Cert, A. Determination of Phenols, Flavones, and Lignans in Virgin Olive Oils by Solid-Phase Extraction and High-Performance Liquid Chromatography with Diode Array Ultraviolet Detection. J. Agric. Food Chem. 2001, 49, 2185–2192. [Google Scholar] [CrossRef]
- Kim, D.-O.; Jeong, S.W.; Lee, C.Y. Antioxidant Capacity of Phenolic Phytochemicals from Various Cultivars of Plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Jerman Klen, T.; Mozetič Vodopivec, B. Optimisation of Olive Oil Phenol Extraction Conditions Using a High-Power Probe Ultrasonication. Food Chem. 2012, 134, 2481–2488. [Google Scholar] [CrossRef] [PubMed]
- Diamantakos, P.; Ioannidis, K.; Papanikolaou, C.; Tsolakou, A.; Rigakou, A.; Melliou, E.; Magiatis, P. A New Definition of the Term “High-Phenolic Olive Oil” Based on Large Scale Statistical Data of Greek Olive Oils Analysed by QNMR. Molecules 2021, 26, 1115. [Google Scholar] [CrossRef] [PubMed]
- Karkoula, E.; Skantzari, A.; Melliou, E.; Magiatis, P. Quantitative Measurement of Major Secoiridoid Derivatives in Olive Oil Using QNMR. Proof of the Artificial Formation of Aldehydic Oleuropein and Ligstroside Aglycon Isomers. J. Agric. Food Chem. 2014, 62, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Diamantakos, P.; Velkou, A.; Brian, K.; Gimisis, T.; Melliou, E.; Magiatis, P. Oleokoronal and Oleomissional: New Major Phenolic Ingredients of Extra Virgin Olive Oil. Olivae 2015, 122, 22–33. [Google Scholar]
- Rigakou, A.; Diamantakos, P.; Melliou, E.; Magiatis, P. S-(E)-Elenolide: A New Constituent of Extra Virgin Olive Oil. J. Sci. Food Agric. 2019, 99, 5319–5326. [Google Scholar] [CrossRef] [PubMed]
- Abuznait, A.H.; Qosa, H.; Busnena, B.A.; El Sayed, K.A.; Kaddoumi, A. Olive-Oil-Derived Oleocanthal Enhances β-Amyloid Clearance as a Potential Neuroprotective Mechanism against Alzheimer’s Disease: In vitro and in Vivo Studies. ACS Chem. Neurosci. 2013, 4, 973–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francisco, V.; Ruiz-Fernandez, C.; Lahera, V.; Lago, F.; Pino, J.; Skaltsounis, L.; Angel Gonzalez-Gay, M.; Mobasheri, A.; Gomez, R.; Scotece, M.; et al. Natural Molecules for Healthy Lifestyles: Oleocanthal from Extra Virgin Olive Oil. J. Agric. Food Chem. 2019, 67, 3845–3853. [Google Scholar] [CrossRef]
- Cui, M.; Chen, B.; Xu, K.; Rigakou, A.; Diamantakos, P.; Melliou, E.; Logothetis, D.E.; Magiatis, P. Activation of Specific Bitter Taste Receptors by Olive Oil Phenolics and Secoiridoids. Sci. Rep. 2021, 11, 22340. [Google Scholar] [CrossRef]
- Segura Palacios, J.M.; Blázquez Sánchez, N.; Rivas Ruiz, F.; Aguilar Bernier, M.; Ramírez López, B.; Sánchez, M.E.F.; de Troya Martín, M. Topical Treatment with Oleocanthal Extract in Reducing Inflammatory Reactions after Photodynamic Therapy: A Prospective Quasi-Experimental Pilot Study. Complement. Ther. Med. 2019, 42, 298–301. [Google Scholar] [CrossRef]
- Filipek, A.; Czerwinska, M.E.; Kiss, A.K.; Wrzosek, M.; Naruszewicz, M. Oleacein Enhances Anti-Inflammatory Activity of Human Macrophages by Increasing CD163 Receptor Expression. Phytomedicine 2015, 22, 1255–1261. [Google Scholar] [CrossRef]
- Naruszewicz, M.; Czerwinska, M.E.; Kiss, A.K. Oleacein. Translation from Mediterranean Diet to Potential Antiatherosclerotic Drug. Curr. Pharm. Des. 2015, 21, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Maugeri, A.; Russo, C.; Musumeci, L.; Navarra, M.; Lombardo, G.E. Oleacein Attenuates Lipopolysaccharide-Induced Inflammation in THP-1-Derived Macrophages by the Inhibition of TLR4/MyD88/NF-ΚB Pathway. Int. J. Mol. Sci. 2022, 23, 1206. [Google Scholar] [CrossRef]
- Menendez, J.A.; Vazquez-Martin, A.; Garcia, R.; Carrasco-Pancorbo, A.; Oliveras-Ferraros, C.; Fernandez-Gutierrez, A.; Segura-Carretero, A. TabAnti-HER2 (ErbB-2) Oncogene Effects of Phenolic Compounds Directly Isolated from Commercial Extra-Virgin Olive Oil (EVOO). BMC Cancer 2008, 23, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanis, D.D.; Scim, S.; Occhipinti, A.; Bertea, C.M.; Costelli, P. Anti-Proliferative Effects of an Extra-Virgin Olive Oil Extract Enriched in Ligstroside Aglycone and Oleocanthal on Human Liver Cancer Cell Lines. Cancers 2019, 20, 1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leri, M.; Oropesa-Nuñez, R.; Canale, C.; Raimondi, S.; Giorgetti, S.; Bruzzone, E.; Bellotti, V.; Stefani, M.; Bucciantini, M. Oleuropein Aglycone: A Polyphenol with Different Targets against Amyloid Toxicity. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1432–1442. [Google Scholar] [CrossRef]
- Brunetti, G.; Di Rosa, G.; Scuto, M.; Leri, M.; Stefani, M.; Schmitz-Linneweber, C.; Calabrese, V.; Saul, N. Healthspan Maintenance and Prevention of Parkinson’s-like Phenotypes with Hydroxytyrosol and Oleuropein Aglycone in C. Elegans. Int. J. Mol. Sci. 2020, 21, 2588. [Google Scholar] [CrossRef] [Green Version]
- Jakobušić Brala, C.; Benčić, D.; Šindrak, Z.; Barbarić, M.; Uršić, S. Labeled Extra Virgin Olive Oil as Food Supplement; Phenolic Compounds in Oils from Some Autochthonous Croatian Olives. Grasas y Aceites 2015, 66, e099. [Google Scholar] [CrossRef]
- Owen, R.W.; Mier, W.; Giacosa, A.; Hull, W.E.; Spiegelhalder, B.; Bartsch, H. Phenolic Compounds and Squalene in Olive Oils: The Concentration and Antioxidant Potential of Total Phenols, Simple Phenols, Secoiridoids, Lignansand Squalene. Food Chem. Toxicol. 2000, 3, 647–659. [Google Scholar] [CrossRef]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, Toxicity, and Clinical Applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- De Pablos, R.M.; Espinosa-Oliva, A.M.; Hornedo-Ortega, R.; Cano, M.; Arguelles, S. Hydroxytyrosol Protects from Aging Process via AMPK and Autophagy; a Review of Its Effects on Cancer, Metabolic Syndrome, Osteoporosis, Immune-Mediated and Neurodegenerative Diseases. Pharmacol. Res. 2019, 143, 58–72. [Google Scholar] [CrossRef]
- Plotnikov, M.B.; Plotnikova, T.M. Tyrosol as a Neuroprotector: Strong Effects of a “Weak” Antioxidant. Curr. Neuropharmacol. 2021, 19, 434–448. [Google Scholar] [CrossRef] [PubMed]
- De, P.; Baltas, M.; Bedos-Belval, F. Cinnamic Acid Derivatives as Anticancer Agents-A Review. Curr. Med. Chem. 2011, 18, 1672–1703. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Qi, M.; Li, P.; Zhan, Y.; Shao, H. Apigenin in Cancer Therapy: Anti-Cancer Effects and Mechanisms of Action. Cell Biosci. 2017, 7, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, M.L.; Vera, D.M.A.; Laiolo, J.; Joray, M.B.; Maccioni, M.; Palacios, S.M.; Molina, G.; Lanza, P.A.; Gancedo, S.; Rumjanek, V.; et al. Mechanism Underlying the Reversal of Drug Resistance in P-Glycoprotein-Expressing Leukemia Cells by Pinoresinol and the Study of a Derivative. Front. Pharmacol. 2017, 8, 205. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Sánchez, A.; Martínez-Ortega, A.J.; Remón-Ruiz, P.J.; Piñar-Gutiérrez, A.; Pereira-Cunill, J.L.; García-Luna, P.P. Therapeutic Properties and Use of Extra Virgin Olive Oil in Clinical Nutrition: A Narrative Review and Literature Update. Nutrients 2022, 14, 1440. [Google Scholar] [CrossRef]
- Nunes, A.; Marto, J.; Goncalves, L.; Martins, A.M.; Fraga, C.; Riberio, H.M. Potential therapeutic of olive oil industry by-products in skin health: A review. Int. J. Food Sci. Technol. 2022, 57, 173–187. [Google Scholar] [CrossRef]
- Torić, J.; Brozovic, A.; Baus Lončar, M.; Jakobušić Brala, C.; Karković Marković, A.; Benčić, Đ.; Barbarić, M. Biological Activity of Phenolic Compounds in Extra Virgin Olive Oils through Their Phenolic Profile and Their Combination with Anticancer Drugs Observed in Human Cervical Carcinoma and Colon Adenocarcinoma Cells. Antioxidants 2020, 9, 453. [Google Scholar] [CrossRef]
- Polini, B.; Digiacomo, M.; Carpi, S.; Bertini, S.; Gado, F.; Saccomanni, G.; Macchia, M.; Nieri, P.; Manera, C.; Fogli, S. Oleocanthal and Oleacein Contribute to the in Vitro Therapeutic Potential of Extra Virgin Oil-Derived Extracts in Non-Melanoma Skin Cancer. Toxicol. In Vitro 2018, 52, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Expasy—SIB Bioinformatics Resource Portal. Available online: https://web.expasy.org/cellosaurus/CVCL_0132 (accessed on 28 March 2022).
- Expasy—SIB Bioinformatics Resource Portal. Available online: https://web.expasy.org/cellosaurus/CVCL_B222 (accessed on 28 March 2022).
- Expasy—SIB Bioinformatics Resource Portal. Available online: https://web.expasy.org/cellosaurus/CVCL_0038 (accessed on 28 March 2022).
- GraphPad Prism. Available online: https://www.graphpad.com/scientific-software/prism/ (accessed on 28 January 2022).





| EVOO (Cultivar) | |||
|---|---|---|---|
| Žižolera | Bjelica | Crnica | |
| TP (mg GAE/kg EVOO ± SD) | 379 ± 15 A | 423 ± 38 A | 344 ± 41 A |
| o-diphenols (mg GAE/kg EVOO ± SD) | 216 ± 9 A | 122 ± 11 B | 137 ± 6 B |
| TF (mg CE/kg EVOO ± SD) | 392 ± 10 A | 230 ± 42 B | 160 ± 4 B |
| Phenolic Compounds/ Secoiridoids (mg/kg EVOO ± SD) | EVOO (Cultivar) | ||
|---|---|---|---|
| Žižolera | Bjelica | Crnica | |
| Oleocanthal | 123 ± 0.1 A | 215 ± 43 A | 157 ± 31 A |
| Oleacein | 216 ± 0.3 A, B | 125 ± 1 A | 329 ± 83 B |
| Oleuropein aglycone | 215 ± 1 A | 75 ± 2 B | 19.4 ± 0.9 C |
| Ligstroside aglycone | 30 ± 1 A | 50 ± 4 B | 7.7 ± 0.3 C |
| Oleokoronal | 113 ± 9 A | 152 ± 9 B | 94.3 ± 5.8 A |
| Oleomissional | 88 ± 2 A | 36 ± 1 B | 96.3 ± 7.8 A |
| S-(E)-elenolide | 1054 ± 20 A | 164 ± 14 B | 514 ± 32 C |
| Hydroxytyrosol | 5.95 ± 0.12 A | 4.85 ± 0.10 B | 1.38 ± 0.17 C |
| Tyrosol | 3.07 ± 0.08 A | 5.47 ± 0.42 B | 1.91 ± 0.13 C |
| Cinnamic acid | 0.90 ± 0.09 A | 0.41 ± 0.02 B | 0.59 ± 0.11 B |
| Pinoresinol | 13.3 ± 0.7 A | 5.40 ± 0.33 B | 6.05 ± 0.57 B |
| Apigenin | 2.53 ± 0.26 A | 0.87 ± 0.01 B | 2.61 ± 0.16 A |
| EVOO-PE Cultivar | OCEIN | OCAL | DTIC | ||||
|---|---|---|---|---|---|---|---|
| Žižolera | Bjelica | Crnica | |||||
| Cell Line | IC | %v/v of EVOO-PE ± SEM | µM | ||||
| A375 | 60 | 0.036 ± 0.002 | 0.057 ± 0.002 | 0.037 ± 0.002 | 101.052 ± 4.610 | 58.488 ± 1.836 | 214.358 ± 13.449 |
| 50 | 0.042 ± 0.002 | 0.067 ± 0.002 | 0.047 ± 0.002 | 112.933 ±4.925 | 67.475 ± 1.863 | 361.716 ± 15,301 | |
| A375M | 60 | 0.355 ± 0.011 | 0.401 ± 0.020 | 0.491 ± 0.052 | ND | ND | 709.780 ± 99.634 |
| 50 | 0.401 ± 0.012 | 0.505 ± 0.034 | 0.546 ± 0.083 | ND | ND | 872.150 ± 62.158 | |
| HaCaT | 60 | 0.126 ± 0.004 | 0.113 ± 0.003 | 0.187 ±0.006 | 103.061 ± 2.086 | 112.827 ± 2.904 | 1137 ± 93.752 |
| 50 | 0.152 ± 0.004 | 0.133 ± 0.003 | 0.233 ± 0.007 | 114.351 ± 2.303 | 130.473 ± 3.215 | 1636.615 ± 122.559 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kugić, A.; Dabelić, S.; Brala, C.J.; Dabelić, N.; Barbarić, M. Extra Virgin Olive Oil Secoiridoids Modulate the Metabolic Activity of Dacarbazine Pre-Treated and Treatment-Naive Melanoma Cells. Molecules 2022, 27, 3310. https://doi.org/10.3390/molecules27103310
Kugić A, Dabelić S, Brala CJ, Dabelić N, Barbarić M. Extra Virgin Olive Oil Secoiridoids Modulate the Metabolic Activity of Dacarbazine Pre-Treated and Treatment-Naive Melanoma Cells. Molecules. 2022; 27(10):3310. https://doi.org/10.3390/molecules27103310
Chicago/Turabian StyleKugić, Azra, Sanja Dabelić, Cvijeta Jakobušić Brala, Nina Dabelić, and Monika Barbarić. 2022. "Extra Virgin Olive Oil Secoiridoids Modulate the Metabolic Activity of Dacarbazine Pre-Treated and Treatment-Naive Melanoma Cells" Molecules 27, no. 10: 3310. https://doi.org/10.3390/molecules27103310
APA StyleKugić, A., Dabelić, S., Brala, C. J., Dabelić, N., & Barbarić, M. (2022). Extra Virgin Olive Oil Secoiridoids Modulate the Metabolic Activity of Dacarbazine Pre-Treated and Treatment-Naive Melanoma Cells. Molecules, 27(10), 3310. https://doi.org/10.3390/molecules27103310