Thermo-Responsive ZnPc-g-TiO2-g-PNIPAM Photocatalysts Sensitized with Phthalocyanines for Water Purification under Visible Light
Abstract
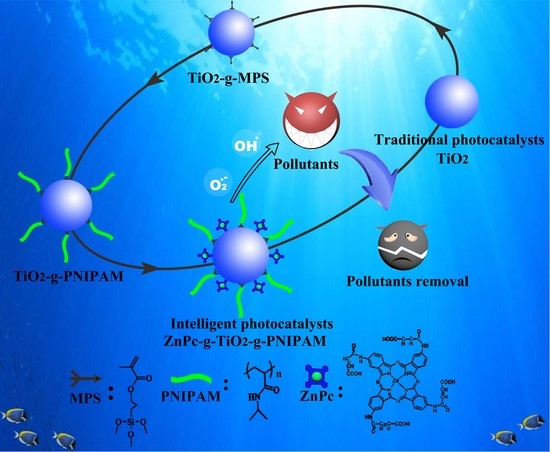
:1. Introduction
2. Results and Discussion
2.1. Photocatalytic Activity
2.2. Mechanism Discussion
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. Surface Modification of TiO2
3.4. PNIPAM Grafting on TiO2
3.5. Immobilizing ZnPc on TiO2-g-PNIPAM
3.6. Photocatalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mahmoodi, N.M.; Taghizadeh, A.; Taghizadeh, M.; Abdi, J. In Situ Deposition of Ag/Agcl on the Surface of Magnetic Metal-Organic Framework Nanocomposite and Its Application for the Visible-Light Photocatalytic Degradation of Rhodamine Dye. J. Hazard. Mater. 2019, 378, 120741. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.M.; Abdi, J.; Taghizadeh, M.; Taghizadeh, A.; Hayati, B.; Shekarchi, A.A.; Vossoughi, M. Activated Carbon/Metal-Organic Framework Nanocomposite: Preparation and Photocatalytic Dye Degradation Mathematical Modeling from Wastewater by Least Squares Support Vector Machine. J. Environ. Manag. 2019, 233, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Eid, C.; Habchi, R.; Miele, P.; Bechelany, M. Recent Progress on Titanium Dioxide Nanomaterials for Photocatalytic Applications. ChemSusChem 2018, 11, 3023–3047. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.; Lim, J.H.; Ko, K.C.; Lee, J.Y. Photocatalytic Activity of TiO2 Nanoparticles: A Theoretical Aspect. J. Mater. Chem. A 2019, 7, 13833–13859. [Google Scholar] [CrossRef]
- Fang, Y.; Zheng, Y.; Fang, T.; Chen, Y.; Zhu, Y.; Liang, Q.; Sheng, H.; Li, Z.; Chen, C.; Wang, X. Photocatalysis: An Overview of Recent Developments and Technological Advancements. Sci. China Chem. 2019, 63, 149–181. [Google Scholar] [CrossRef] [Green Version]
- Sadeghfar, F.; Zalipour, Z.; Taghizadeh, M.; Taghizadeh, A.; Ghaedi, M. Photodegradation Processes. Interface Sci. Technol. 2021, 32, 55–124. [Google Scholar]
- Taghizadeh, A.; Taghizadeh, M.; Sabzehmeidani, M.M.; Sadeghfar, F.; Ghaedi, M. Electronic Structure: From Basic Principles to Photocatalysis. Interface Sci. Technol. 2021, 32, 1–53. [Google Scholar]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Kim, B.C.; Jeong, E.; Kim, E.; Hong, S.W. Bio-Organic–Inorganic Hybrid Photocatalyst, TiO2 and Glucose Oxidase Composite for Enhancing Antibacterial Performance in Aqueous Environments. Appl. Catal. B Environ. 2019, 242, 194–201. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Zhou, J.; Jin, W.; Chen, W. Tunability of Photo-Catalytic Selectivity of B-Doped Anatase TiO2 Microspheres in the Visible Light. Dye. Pigment. 2018, 156, 213–218. [Google Scholar] [CrossRef]
- Yu, X.; Liu, S.; Yu, J. Superparamagnetic Γ-Fe2O3@SiO2@TiO2 Composite Microspheres with Superior Photocatalytic Properties. Appl. Catal. B Environ. 2011, 104, 12–20. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, T.F.; Feng, D.; Tian, J.; Wang, K.; Qin, J.; Zhang, Q.; Chen, Y.P.; Bosch, M.; Zou, L.; et al. A Single Crystalline Porphyrinic Titanium Metal-Organic Framework. Chem. Sci. 2015, 6, 3926–3930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, W.; Xu, T.; Wang, Y.; Hu, H.; Li, N.; Jiang, X.; Chen, W. Synergistic Photocatalytic Properties and Mechanism of G-C3N4 Coupled with Zinc Phthalocyanine Catalyst under Visible Light Irradiation. Appl. Catal. B Environ. 2016, 180, 20–28. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, B.; Mu, J.; Zhang, M.; Zhang, P.; Zhang, Z.; Wang, J.; Zhang, X.; Sun, Y.; Shao, C.; et al. Iron Phthalocyanine/TiO2 Nanofiber Heterostructures with Enhanced Visible Photocatalytic Activity Assisted with H2O2. J. Hazard. Mater. 2012, 219, 156–163. [Google Scholar] [CrossRef]
- Gong, Z.; Li, S.; Han, W.; Wang, J.; Ma, J.; Zhang, X. Recyclable Graphene Oxide Grafted with Poly(N-Isopropylacrylamide) and Its Enhanced Selective Adsorption for Phenols. Appl. Surf. Sci. 2016, 362, 459–468. [Google Scholar] [CrossRef]
- Tanjim, M.; Rahman, M.A.; Rahman, M.M.; Minami, H.; Hoque, S.M.; Sharafat, M.K.; Gafur, M.A.; Ahmad, H. Mesoporous Magnetic Silica Particles Modified with Stimuli-Responsive P(Nipam-Dma) Valve for Controlled Loading and Release of Biologically Active Molecules. Soft Matter 2018, 14, 5469–5479. [Google Scholar] [CrossRef]
- Duan, Y.; Ma, J.; Liu, J.; Qiang, L.; Xue, J. Facile Synthesis of Thermo-Responsive TiO2/Pnipam Composite with Switchable Photocatalytic Performance. Fiber Polym. 2020, 21, 717–723. [Google Scholar] [CrossRef]
- Yao, B.; Peng, C.; Zhang, W.; Zhang, Q.; Niu, J.; Zhao, J. A Novel Fe(III) Porphyrin-Conjugated TiO2 Visible-Light Photocatalyst. Appl. Catal. B Environ. 2015, 174, 77–84. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Yu, M.; Wang, C.; Li, J. The Highly Efficient and Stable Cu, Co, Zn-Porphyrin–Tio2 Photocatalysts with Heterojunction by Using Fashioned One-Step Method. Dye. Pigment. 2017, 136, 648–656. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Dhodamani, A.G.; Suzuki, N.; Terashima, C.; Fujishima, A.; Mathe, V.L. Enhanced Photocatalytic Performance of Ultrasound Treated Go/TiO2 Composite for Photocatalytic Degradation of Salicylic Acid under Sunlight Illumination. Ultrason. Sonochem. 2020, 61, 104849. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Tang, D.; Lv, H.; Zhang, W.; Li, W. Surface-Initiated Atrp to Modify Zno Nanoparticles with Poly(N -Isopropylacrylamide): Temperature-Controlled Switching of Photocatalysis. J. Alloys Compd. 2017, 691, 185–194. [Google Scholar] [CrossRef]
- Phongamwong, T.; Donphai, W.; Prasitchoke, P.; Rameshan, C.; Barrabés, N.; Klysubun, W.; Rupprechter, G.; Chareonpanich, M. Novel Visible-Light-Sensitized Chl-Mg/P25 Catalysts for Photocatalytic Degradation of Rhodamine B. Appl. Catal. B Environ. 2017, 207, 326–334. [Google Scholar] [CrossRef]
- Wu, C.; Yin, M.; Zhang, R.; Li, Z.; Zou, Z.; Li, Z. Further Studies of Photodegradation and Photocatalytic Hydrogen Production over Nafion-Coated Pt/P25 Sensitized by Rhodamine B. Int. J. Hydrogen Energy 2020, 45, 22700–22710. [Google Scholar] [CrossRef]
- Bai, X.; Du, Y.; Hu, X.; He, Y.; He, C.; Liu, E.; Fan, J. Synergy Removal of Cr(VI) and Organic Pollutants over Rp-MoS2/RGO Photocatalyst. Appl. Catal. B Environ. 2018, 239, 204–213. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Cui, X.; Liang, C.; Xing, G.; Duan, Q. Construction of a Recyclable Dual-Responsive TiO2-Based Photocatalyst Modified with ZnIn2S4 Nanosheets and Zinc Phthalocyanine for Cr(VI) Reduction under Visible Light. Chem. Eng. J. 2021, 417, 129332. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Q.; Wu, Y.; Yuan, Y.; Liu, Y. Investigation of Nanoscale Zerovalent Iron-Based Magnetic and Thermal Dual-Responsive Composite Materials for the Removal and Detection of Phenols. Chemosphere 2018, 195, 472–482. [Google Scholar] [CrossRef]
- Dai, Z.; Li, D.; Chi, L.; Li, Y.; Gao, B.; Qiu, N.; Duan, Q.; Li, Y. Preparation of Porphyrin Sensitized Three Layers Magnetic Nanocomposite Fe3O4@SiO2@TiO2 as an Efficient Photocatalyst. Mater. Lett. 2019, 241, 239–242. [Google Scholar] [CrossRef]
- Lu, X.; Che, W.; Hu, X.; Wang, Y.; Zhang, A.; Deng, F.; Luo, S.; Dionysiou, D.D. The Facile Fabrication of Novel Visible-Light-Driven Z-Scheme CuInS2/Bi2Wo6 Heterojunction with Intimate Interface Contact by in Situ Hydrothermal Growth Strategy for Extraordinary Photocatalytic Performance. Chem. Eng. J. 2019, 356, 819–829. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, D.; Li, Y.; Liu, Y.; Duan, Q.; Kakuchi, T. Synthesis of water-soluble and thermoresponsive phthalocyanine ended block copolymers as potential photosensitizer. Dyes Pigment. 2017, 142, 88–99. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, B.; Liu, C.; Cui, X.; Li, Y.; Duan, Q. Thermo-Responsive ZnPc-g-TiO2-g-PNIPAM Photocatalysts Sensitized with Phthalocyanines for Water Purification under Visible Light. Molecules 2022, 27, 3330. https://doi.org/10.3390/molecules27103330
Mao B, Liu C, Cui X, Li Y, Duan Q. Thermo-Responsive ZnPc-g-TiO2-g-PNIPAM Photocatalysts Sensitized with Phthalocyanines for Water Purification under Visible Light. Molecules. 2022; 27(10):3330. https://doi.org/10.3390/molecules27103330
Chicago/Turabian StyleMao, Bingxin, Cong Liu, Xu Cui, Yanhui Li, and Qian Duan. 2022. "Thermo-Responsive ZnPc-g-TiO2-g-PNIPAM Photocatalysts Sensitized with Phthalocyanines for Water Purification under Visible Light" Molecules 27, no. 10: 3330. https://doi.org/10.3390/molecules27103330
APA StyleMao, B., Liu, C., Cui, X., Li, Y., & Duan, Q. (2022). Thermo-Responsive ZnPc-g-TiO2-g-PNIPAM Photocatalysts Sensitized with Phthalocyanines for Water Purification under Visible Light. Molecules, 27(10), 3330. https://doi.org/10.3390/molecules27103330