Overview of Allelopathic Potential of Lemna minor L. Obtained from a Shallow Eutrophic Lake
Abstract
:1. Introduction
1.1. Allelopathic Potential of Lemna minor L.
1.2. Models of Lemna minor L. and Cladophora glomerata (L.) Kütz Population Formation: Interactions between Species
- Do Lemna minor and Cladophora glomerata secrete allelopathic substances in coexistence?
- Does the allelopathic potential occur in competing interactions?
2. Chemical Composition of L. minor and C. glomerata
3. Results
3.1. Chemical Composition Analysis of L. minor and C. glomerata
3.2. Analysis of the Morphological Features of Cladophora glomerata and Lemna minor
3.3. The Size of the Patches of L. minor and C. glomerata in the Littoral Zone
3.4. The Content of Phenolic Acids in L. minor and C. glomerata
3.5. Relationships between the Parameters of Dry Mass of L. minor and C. glomerata and the Effect of Polyphenol Concentration and Time
4. Discussion
5. Conclusions
6. Materials and Methods
6.1. Raw Material Collection and Identification
6.2. Element Analysis of Lemna minor and Cladophora glomerata Raw Material
6.3. Ultrasound-Assisted Extraction (UAE)
6.4. Determination of Total Phenolic Compounds in Extracts
6.5. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Falińska, K. Ekologia Roślin; PWN: Warsaw, Poland, 2012; pp. 29–32. ISBN 9788301142223. [Google Scholar]
- Kufel, L.; Strzałek, M.; Konieczna, A.; Izdebska, K. The effect of Stratiotes aloides L. and nutrients on the growth rate of Lemna minor L. Aquat. Bot. 2010, 92, 168–172. [Google Scholar] [CrossRef]
- Gross, E.M. Allelopathy of Aquatic Autotrophs. Crit. Rev. Plant Sci. 2010, 22, 313–339. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Bhadha, J.H.; Rott, P.; Beuzelin, J.M.; Kanissery, R. Investigating the use of aquatic weeds as biopesticides towards promoting sustainable agriculture. PLoS ONE 2021, 15, e0237258. [Google Scholar] [CrossRef]
- Bich, T.T.N.; Kato-Noguchi, H. Allelopathic potential of two aquatic plants, duckweed (Lemna minor L.) and water lettuce (Pistia stratiotes L.), on terrestrial plant species. Aquat. Bot. 2012, 103, 30–36. [Google Scholar] [CrossRef]
- Gopal, B.; Goel, U. The Botanical Review: School of Environmental Sciences; Jawaharlal Nehru University: New Delhi, India, 1993; pp. 171–181. [Google Scholar]
- Einheling, F.A.; Leather, G.R.; Hobbs, L.L. Use of Lemna minor L. as a bioassay in allelopathy. J. Chem. Ecol. 1985, 11, 65–72. [Google Scholar] [CrossRef]
- Akbulut, G.B.; Turhan, D.Ö.; YİĞİT, E. Alleviation of Everzol Red LFB Toxicity in Duclweed (Lemna minor L.) by Exogenous Salicylic Acid. KSU J. Agric. Nat. 2020, 23, 876–884. [Google Scholar]
- Gülçin, I.; Kireçci, E.; Akkemik, E.; Topal, F.; Hisar, O. Antioxidant, antibacterial, and anticandidal activities of an aquatic plant: Duckweed (Lemna minor L. Lemnaceae). Turk. J. Biol. 2010, 34, 175–188. [Google Scholar]
- Al-Snafi, A.E. Lemna minor: Traditional Uses, Chemical Constituents and Pharmacological Effects—A Review. IOSR J. Pharm. 2019, 9, 6–11. [Google Scholar]
- Vladimirova, I.N.; Georgiyants, V.A. Biologically Active Compounds from Lemna minor S. F. Gray. Pharm. Chem. J. 2014, 47, 599–601. [Google Scholar] [CrossRef]
- Radulović, O.; Stanković, S.; Stanojević, O.; Vujčić, Z.; Dojnov, B.; Trifunović-Momčilov, M.; Marković, M. Antioxidative Responses of Duckweed (Lemna minor L.) to Phenol and Rhizosphere-Associated Bacterial Strain Hafnia paralvei C32-106/3. Antioxidants 2021, 10, 1719. [Google Scholar] [CrossRef]
- Radulović, O.; Stanković, S.; Uzelac, B.; Tadić, V.; Trifunović-Momčilov, M.; Lozo, J.; Marković, M. Phenol Removal Capacity of the Common Duckweed (Lemna minor L.) and Six Phenol-Resistant Bacterial Strains from Its Rhizosphere: In Vitro Evaluation at High Phenol Concentrations. Plants 2020, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Stom, D.I.; Roth, R. Some effects of polyphenols on aquatic plants: I. Toxicity of phenols in aquatic plants. Bull. Environ. Contam. Toxicol. 1981, 27, 332–337. [Google Scholar] [CrossRef]
- Gałczyńska, M.; Mańkowska, N.; Milke, J.; Buśko, M. Possibilities and limitations of using Lemna minor, Hydrocharis morsus-ranae and Ceratophyllum demersum in removing metals with contaminates water. J. Water Land Dev. 2019, 40, 161–173. [Google Scholar] [CrossRef]
- Bog, M.; Appenroth, K.J.; Sree, S. Key to the determination of taxa of Lemnaceae: An update. Nord. J. Bot. 2020, 38, e02658. [Google Scholar] [CrossRef]
- Frédéric, M.; Lasfar, S.; Louise, M.; Adelkrim, A. Comprehensive modeling of mat density effect on duckweed (Lemna minor) growth under controlled eutrophication. Water Res. 2006, 40, 2901–2910. [Google Scholar] [CrossRef]
- Lemon, G.D.; Posluszny, U.; Husband, B.C. Potential and realized rates of vegetative reproduction in Spirodela polyrhiza, Lemna minor, and Wolffia borealis. Aquat. Bot. 2001, 70, 79–87. [Google Scholar] [CrossRef]
- Appenroth, K.J.; Borisjuk, N. Telling Duckweed Apart: Genotyping Technologies for the Lemnaceae. Chin. J. Appl. Environ. Biol. 2013, 19, 1–10. [Google Scholar]
- Parr, B.L.; Perkins, R.G.; Mason, C.F. Recustion in photosynthetic efficiency of Cladophora glomerate, induced by overlying canopies of Lemna spp. Water Res. 2002, 36, 1735–1742. [Google Scholar] [CrossRef]
- Hasan, M.R.; Chakrabarti, R. Use of Algae ad Aquatic Macrophytes as Feed in Small-Scale Aquaculture: A Review; FAO: Roma, Italy, 2009; pp. 29–43. ISBN 9789251064207. [Google Scholar]
- Pastierova, A.; Sirotiak, M.; Fiala, J. Comprehensive Study of Duckweed Cultivation and Growth Conditions under Controlled Eutrophication. Res. Pap. Fac. Mater. Sci. Technol. Slovak Univ. Technol. 2015, 23, 103–107. [Google Scholar]
- Palag, G.; Selamoglu, Z.; Caglar, M. Algae of Lemna minor L. growing in natural habitat and aquarium. Iran. J. Fish. Sci. 2019, 18, 1076–1082. [Google Scholar]
- Pikosz, M.; Messyasz, B. Characteristics of Cladophora and coexisting filamentous algae in relation to environmental factors in freshwater ecosystems in Poland. Oceanol. Hydrobiol. Stud. 2016, 45, 202–215. [Google Scholar] [CrossRef]
- Pikosz, M.; Messyasz, B.; Gąbka, M. Functional structure of algal mat (Cladophora glomerata) in a freshwater in western Poland. Ecol. Indic. 2017, 17, 1–9. [Google Scholar] [CrossRef]
- Messyasz, B.; Pikosz, M.; Treska, E. Macroalgae and Their Distribution. In Algae Biomass: Characteristics and Applications Biology of Freshwater; Chojnacka, K., Wieczorek, P.P., Schroeder, G., Michalak, I., Eds.; Developments in Applied Phycology: Cham, Switzerland, 2018; pp. 17–31. ISBN 9783319747033. [Google Scholar]
- Schroeder, G.; Łęska, B.; Messyasz, B.; Pikosz, M. Extraction of macroalgae biomass for cosmetics industry. Przemysł Chem. 2015, 94, 405–407. [Google Scholar]
- Pikosz, M.; Messyasz, B. Composition and seasonal changes in filamentous algae in floating mats. Oceanol. Hydrobiol. Stud. 2015, 44, 273–281. [Google Scholar] [CrossRef]
- Schroeder, G.; Łęska, B.; Fabrowska, J.; Messyasz, B. Analysis of Green Algae Extracts. In Marine Algae Extracts; Kim, S.K., Chojnacka, K., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 79–100. ISBN 9783527679577. [Google Scholar]
- Ozimek, T. Aspects of the ecology of a filamentous alga in a eutrophicated lake. Hydrobiologia 1990, 191, 23–27. [Google Scholar] [CrossRef]
- Kartal, M.; Orhan, I.; Abu-Asaker, M.; Senol, F.S.; Atici, T.; Sener, B. Antioxidant and Anticholinesterase Assets and Liquid Chromatography-Mass Spectrometry Preface of Various Fresh-Water and Marine Macroalgae. Pharmacogn. Mag. 2009, 5, 291–297. [Google Scholar]
- Petrova-Tacheva, V.; Ivanov, V.; Atanasov, A. Lemna minor, L. As a Source of Antioxidants. Trakia J. Sci. 2020, 18, 157–162. [Google Scholar] [CrossRef]
- Gipson, A.B.; Morton, K.J.; Rhee, R.J.; Simo, S.; Clayton, J.A.; Perrett, M.E.; Binley, C.G.; Jensen, E.L.; Oakes, D.L.; Rouhier, M.F.; et al. Disruptions in valine degradation affect seed development and germination in Arabidopsis. Plant J. 2017, 90, 1029–1039. [Google Scholar] [CrossRef]
- Kárpáti, V.; Pomogyi, P. Accumulation and release of nutrients by aquatic macrophytes. Symp. Biol. Hung. 1979, 19, 33–42. [Google Scholar]
- Qiu, X.M.; Sun, Y.Y.; Ye, X.Y.; Li, Z.G. Signaling Role of Glutamate in Plants. Front. Plant Sci. 2020, 10, 1743. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, D.; Rendon, G.A.; Stewart, G.R. The control of glutamine synthetase level in Lemna minor L. Planta 1975, 125, 201–211. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Ding, N.Z. Plant Unsaturated Fatty Acids: Multiple Roles in Stress Response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef]
- Parsa, M.; Jalilzadeh, H.; Pazoki, M.; Ghasemzadeh, R.; Abduli, M. Hydrothermal liquefaction of Gracilaria gracilis and Cladophora glomerata macri-algae for biocrude production. Bioresour. Technol. 2018, 250, 26–34. [Google Scholar] [CrossRef] [PubMed]
- El-Adl, M.F.; Deyab, M.A.; El-Shanawany, R.S.; Abu Ahmed, S.E. Fatty acids of Cladophora glomerata and Chaetomorpha vieillardii (Cladophoraceae) of different niches inhibit the pathogenic microbial growth. Aquat. Bot. 2022, 176, 103461. [Google Scholar] [CrossRef]
- Pikosz, M.; Czerwik-Marcinkowska, J.; Messyasz, B. The effect of Cladophora glomerate exudates on the amino acid composition of Cladophora fracta and Rhizoclonium sp. Open Chem. 2019, 17, 1–12. [Google Scholar] [CrossRef]
- Gross, E.M.; Erhard, D.; Iványi, E. Allelopathic activity of Ceratophyllum demersum L. and Najas marina ssp. Intermedia (Wolfgang) Casper. Hydrobiol. 2003, 506, 583–589. [Google Scholar]
- Fabrowska, J.; Messyasz, B.; Pankiewicz, R.; Wilińska, P.; Łęska, B. Seasonal differences in the content of phenols and pigments in thallii of freshwater Cladophora glomerata and its habitat. Water Res. 2018, 135, 66–74. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–179. [Google Scholar]
- Oksanen, J. Multivariate Analysis of Ecological Communities in R: Vegan Tutorial; R Package Version 2.0–1; Helsinki University Press: Helsinki, Finland, 2011; p. 43. [Google Scholar]
- Hastie, T.J.; Tibshirani, R.J. Generalized Additive Models; Chapman & Hall: London, UK, 1990; p. 423. [Google Scholar]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003; p. 269. [Google Scholar]








| The Name of the Amino Acid | Amino Acid Amount (%) |
|---|---|
| Glutamic acid | 13.53 |
| Leucine | 10.27 |
| Aspartic acid | 9.89 |
| Alanine | 7.88 |
| Valine | 7.67 |
| Glycine | 7.36 |
| Phenylalanine | 6.28 |
| Lysine | 6.2 |
| Isoleucine | 5.89 |
| Threonine | 5.08 |
| Proline | 4.88 |
| Arginine | 4.67 |
| Serine | 4.05 |
| Tyrosine | 2.76 |
| Histidine | 2.32 |
| Tryptophan | 0.85 |
| Methionine | 0.39 |
| Cystine | Small amounts |
| Name Chemical Element | The Amount of Chemical Element (mg/100 g) [10,11] | Dry Mass (%) [34] |
|---|---|---|
| Calcium | 4990 | 0.18 |
| Potassium | 2495 | 1.53 |
| Silicon | 2495 | - |
| Sodium | 1870 | 0.02 |
| Manganese | 935 | 0.03 |
| Iron | 934 | 0.06 |
| Phosphorus | 515 | 0.83 |
| Magnesium | 155 | 1.92 |
| Aluminum | 0.93 | - |
| Nickel | 0.93 | - |
| Copper | 0.78 | - |
| Lead | 0.03 | - |
| Molybdenum | 0.02 | - |
| Zinc | 0.01 | 0.05 |
| Nitrogen | - | 8.74 |
| Chemical Composition | Content of Chemical Composition |
|---|---|
| Carbohydrate | 34.7 ± 0.4 |
| C (wt.%) | 31.33 |
| O (wt.%) | 30.67 |
| Protein | 26.3 ± 0.36 |
| H (wt.%) | 4.99 |
| N (wt.%) | 4.9 |
| Lipid | 2.4 ± 0.15 |
| S (wt.%) | 1.99 |
| Mineral Metal Content | Dry Mass (g·kg−1) |
|---|---|
| Potassium | 94.1 |
| Calcium | 56.4 |
| Iron | 26.5 |
| Aluminum | 23.1 |
| Magnesium | 13.5 |
| Sodium | 11.5 |
| Chrome | 0.247 |
| Chemical Compounds | Content of Chemical Compounds (%) |
|---|---|
| n-Hexadecanoic acid (palmitic acid) (C16:0) | 45.06 |
| Hexanedioic acid, mono (2-ethylhexyl) ester | 19.51 |
| Tetradecanoic acid (myristic acid) (C14:0) | 14.55 |
| 2–Pentadecanone, 6,10,14-trimethyl (C15:0) | 10.53 |
| Tetradecanoic acid, 12-methyl ester | 4.48 |
| Hexadecanoic acid, ethyl ester (ethyl palmitate) | 2.92 |
| 9,12 Octadecadienoyl chloride (linoleoyl chloride) | 2.92 |
| Element | The Element Content (mg·kg−1) |
|---|---|
| Potassium | 21,940 |
| Calcium | 13,020 |
| Phosphorus | 5411 |
| Magnesium | 2455 |
| Nitrogen | 874 |
| Iron | 485 |
| Boron | 446 |
| Copper | 328 |
| Zinc | 94.9 |
| Manganese | 68.4 |
| Element | The Element Content (g∙g−1 of Dry Mass) |
|---|---|
| Calcium | 148.2 ± 3.1 |
| Potassium | 18.24 ± 0.16 |
| Magnesium | 3.48 ± 0.02 |
| Lead | 0.75 ± 0.05 |
| Arsenic | 0.51 ± 0.04 |
| Sodium | 0.45 ± 0.02 |
| Iron | 0.21 ± 0.01 |
| Nickel | 0.12 ± 0.03 |
| Manganese | 0.08 ± 0.03 |
| Cadmium | 0.06 ± 0.01 |
| Copper | 0.05 ± 0.01 |
| Zinc | 0.03 ± 0.01 |
| Cobalt | 0.01 + 0.001 |
| Chrome | 0.01 + 0.001 |
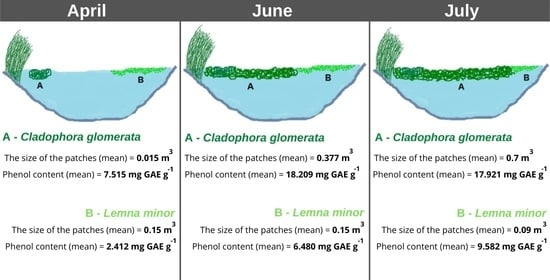
| Date | Cladophora glomerata | |||
|---|---|---|---|---|
| Mean | Minimum | Maximum | Standard Deviation | |
| 30 April 2021 | 0.015 | 0.01 | 0.02 | 0.005 |
| 14 June 2021 | 0.377 | 0.32 | 0.42 | 0.051 |
| 17 July 2021 | 0.700 | 0.65 | 0.76 | 0.056 |
| Lemna minor | ||||
| 30 April 2021 | 0.15 | 0.10 | 0.20 | 0.050 |
| 14 June 2021 | 0.15 | 0.10 | 0.19 | 0.046 |
| 17 July 2021 | 0.09 | 0.08 | 0.10 | 0.010 |
| Date | Cladophora glomerata | |||
|---|---|---|---|---|
| Mean | Minimum | Maximum | Standard Deviation | |
| 30 April 2021 | 7.515 | 7.418 | 7.601 | 0.092 |
| 14 June 2021 | 18.209 | 17.934 | 18.459 | 0.263 |
| 17 July 2021 | 17.921 | 17.306 | 18.269 | 0.534 |
| Lemna minor (from Oporzyńskie Lake) | ||||
| 30 April 2021 | 2.412 | 2.111 | 2.892 | 0.420 |
| 14 June 2021 | 6.480 | 5.987 | 6.876 | 0.452 |
| 17 July 2021 | 9.582 | 9.423 | 9.816 | 0.201 |
| Lemna minor (from breeding) | ||||
| 30 April 2021 | 1.902 | 1.794 | 2.006 | 0.106 |
| 14 June 2021 | 2.035 | 2.014 | 2.052 | 0.019 |
| 17 July 2021 | 2.034 | 2.029 | 2.042 | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gostyńska, J.; Pankiewicz, R.; Romanowska-Duda, Z.; Messyasz, B. Overview of Allelopathic Potential of Lemna minor L. Obtained from a Shallow Eutrophic Lake. Molecules 2022, 27, 3428. https://doi.org/10.3390/molecules27113428
Gostyńska J, Pankiewicz R, Romanowska-Duda Z, Messyasz B. Overview of Allelopathic Potential of Lemna minor L. Obtained from a Shallow Eutrophic Lake. Molecules. 2022; 27(11):3428. https://doi.org/10.3390/molecules27113428
Chicago/Turabian StyleGostyńska, Julia, Radosław Pankiewicz, Zdzisława Romanowska-Duda, and Beata Messyasz. 2022. "Overview of Allelopathic Potential of Lemna minor L. Obtained from a Shallow Eutrophic Lake" Molecules 27, no. 11: 3428. https://doi.org/10.3390/molecules27113428
APA StyleGostyńska, J., Pankiewicz, R., Romanowska-Duda, Z., & Messyasz, B. (2022). Overview of Allelopathic Potential of Lemna minor L. Obtained from a Shallow Eutrophic Lake. Molecules, 27(11), 3428. https://doi.org/10.3390/molecules27113428