Vanadium-Substituted Dawson-Type Polyoxometalate–TiO2 Nanowire Composite Film as Advanced Cathode Material for Bifunctional Electrochromic Energy-Storage Devices
Abstract
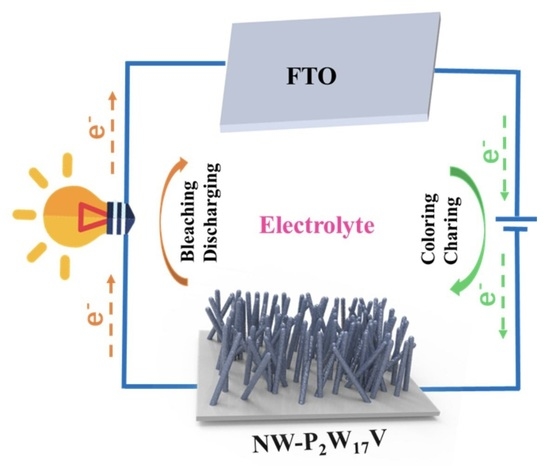
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of the Composite Films
2.3. Characterisation
3. Results and Discussion
3.1. Characterisation of the NW−P2W17V and FTO−P2W17V Materials
3.2. EC Performance
3.3. Energy-Storage Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chang, P.; Mei, H.; Zhang, M.G.; Zhao, Y.; Wang, X.; Cheng, L.F.; Zhang, L.T. 3D printed electrochromic supercapacitors with ultrahigh mechanical strength and energy density. Small 2021, 17, 2102639. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.J.; Zheng, N.; Antonietti, M. Advanced nanomaterials for energy conversion and storage: Current status and future opportunities. Nanoscale 2021, 13, 9904–9907. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.L.; Cui, Y.; Gogotsi, Y. Energy storage: The future enabled by nanomaterials. Science 2019, 366, 8285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, K.; Zhang, Q.Q.; Hao, Z.D.; Tang, Y.H.; Wang, H.; Liu, J.B.; Yan, H. Integrated electrochromic supercapacitors with visual energy levels boosted by coating onto carbon nanotube conductive networks. Sol. Energy Mater. Sol. Cells 2020, 206, 110330. [Google Scholar] [CrossRef]
- Koo, B.R.; Jo, M.H.; Kim, K.H.; Ahn, H.J. Amorphous-quantized WO3·H2O films as novel flexible electrode for advanced eletrchromic energy storage devices. Chem. Eng. J. 2021, 424, 130383. [Google Scholar] [CrossRef]
- Ling, H.; Wu, J.C.; Su, F.Y.; Tian, Y.Q.; Liu, Y.J. Automatic light-adjusting electrochromic device powered by perovskite solar cell. Nat. Commun. 2021, 12, 1010. [Google Scholar] [CrossRef]
- Koo, B.R.; Jo, M.H.; Kim, K.H.; Ahn, H.J. Multifunctional electrochromic energy storage devices by chemical cross-linking: Impact of a WO3·H2O nanoparticle-embedded chitosan thin film on amorphous WO3 films. NPG Asia Mater. 2020, 12, 10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.P.; Zhu, F.; Wang, F.X.; Wang, J.H.; Dong, R.H.; Zhuang, X.D.; Schmidt, O.G.; Feng, X.L. Stimulus-responsive micro-supercapacitors with ultrahigh energy density and reversible electrochromic window. Adv. Mater. 2017, 29, 1604491. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.Y.; Li, W.J.; Song, Y.; Li, Y.; Zhao, J.P. Visualization electrochromic-supercapacitor device based on porous Co doped NiO films. J. Alloys Compd. 2021, 857, 158087. [Google Scholar] [CrossRef]
- Rai, V.; Singh, R.S.; Blackwood, D.J.; Zhili, D. A review on recent advances in electrochromic devices: A material approach. Adv. Eng. Mater. 2020, 22, 2000082. [Google Scholar] [CrossRef]
- Maity, S.; Vannathan, A.A.; Kumar, K.; Das, P.P.; Mal, S.S. Enhanced power density of graphene oxide phosphotetradecavanadate nanohybrid for supercapacitor electrode. J. Mater. Eng. Perform. 2021, 30, 1371–1377. [Google Scholar] [CrossRef]
- Kim, J.; Rémond, M.; Kim, D.; Jang, H.; Kim, E. Electrochromic conjugated polymers for multifunctional smart windows with integrative functionalities. Adv. Mater. Technol. 2020, 5, 1900890. [Google Scholar] [CrossRef]
- Wang, C.L.; Rong, S.; Zhao, Y.Q.; Wang, X.M.; Ma, H.Y. Three-dimensional supramolecular crystalline materials based on Kegin-based polyoxometalates and 1,2-Bis (4-pyridyl) ethylene for supercapacitor electrodes. Transition Met. Chem. 2021, 46, 335–343. [Google Scholar] [CrossRef]
- Wang, S.M.; Wang, Y.H.; Wang, T.; Han, Z.B.; Cho, C.; Kim, E. Charge-Balancing Redox Mediators for High Color Contrast Electrochromism on Polyoxometalates. Adv. Mater. Technol. 2020, 5, 2000326. [Google Scholar] [CrossRef]
- Fu, Z.J.; Qu, Z.Y.; Yu, T.; Bi, L.H. Study on electrochromic-fluorescence switching performance of film based on silicomolybdotungstate and silica nanoparticles doped with negative charged dye. J. Electroanal. Chem. 2019, 855, 113623. [Google Scholar] [CrossRef]
- Li, Y.T.; Yan, L.T.; Zhang, J.; Xu, M.Y.; Zhu, Y.Y. Preparation of graphene/W18O49 nanorod composites and their application in electrochromic performance. J. Mater. Sci. Mater. Electron. 2019, 30, 20181–20188. [Google Scholar] [CrossRef]
- Truong, Q.D.; Le, T.S.; Hoa, T.H. Ultrathin TiO2 rutile nanowires enable reversible Mg-ion intercalation. Mater. Lett. 2019, 254, 357–360. [Google Scholar] [CrossRef]
- Khanna, S.; Marathey, P.; Paneliya, S.; Chaudhari, R.; Vora, J. Fabrication of rutile—TiO2 nanowire on shape memory alloy: A potential material for energy storage application. Mater. Today Proc. 2021, 46, 335–343. [Google Scholar] [CrossRef]
- Ji, Q.; Hou, Y.F.; Wei, S.X.; Liu, Y.; Du, P.; Luo, L.H.; Li, W.P. Excellent energy storage performance in bilayer composites combining aligned TiO2 nanoarray and random TiO2 nanowires with Poly(vinylidene fluoride). J. Phys. Chem. C. 2020, 124, 2864–2871. [Google Scholar] [CrossRef]
- Lv, H.M.; Li, N.; Zhang, H.C.; Tian, Y.L.; Zhang, H.M.; Zhang, X.; Qu, H.Y.; Liu, C.; Jia, C.Y.; Zhao, J.P.; et al. Transferable TiO2 nanotubes membranes formed via anodization and their application in transparent. Sol. Energy Mater. Sol. Cells 2016, 150, 57–64. [Google Scholar] [CrossRef]
- Zhang, S.H.; Chen, S.; Yang, F.; Hu, F.; Yan, B.; Gu, Y.C.; Jiang, H.; Cao, Y.; Xiang, M. High-performance electrochromic device based on novel polyaniline nanofibers wrapped antimony-doped tin oxide/TiO2 nanorods. Org. Electron. 2019, 65, 341–348. [Google Scholar] [CrossRef]
- Qu, X.S.; Feng, H.; Liu, S.P.; Yang, Y.Y.; Ma, C. Enhanced electrochromic performance of nanocomposite film based on Preyssler-type polyoxometalate and TiO2 nanowires. Inorg. Chem. Commun. 2018, 98, 174–179. [Google Scholar] [CrossRef]
- Qu, X.S.; Ma, C.; Fu, Y.; Liu, S.P.; Wang, J.; Yang, Y.Y. Construction of a vertically arrayed three-dimensional composite structure as a high coloration efficiency electrochromic film. New J. Chem. 2020, 44, 4177–4184. [Google Scholar] [CrossRef]
- Qu, X.S.; Fu, Y.; Ma, C.; Yang, Y.Y.; Shi, D.; Chu, D.X.; Yu, X.Y. Bifunctional electrochromic-energy storage materials with ehanced performance obtained by hybridizing TiO2 nanowires with POMs. New J. Chem. 2020, 44, 15475–15482. [Google Scholar] [CrossRef]
- Harmalker, S.P.; Leparulo, M.A.; Pope, M.T. Mixed-valence chemistry of adjacent vanadium centers in heteropolytungstate anions synthesis and electronic structures of mono-, di-, and trisubstituted derivatives of α–[P2W18O62]6-. J. Am. Chem. Soc. 1983, 105, 4286–4292. [Google Scholar] [CrossRef]
- Abbessi, M.; Contant, R.; Thouvenot, R.; Hervé, G. Dawson type heteropolyanions. 1. Multinuclear (31P,51V,183W) NMR structural investigations of octadeca (molybdotungstovanado) diphosphates α-l,2,3-[P2MM’2W15O62]n- (M, M’=Mo, V, W): Syntheses of new related compounds. Inorg. Chem. 1991, 30, 1695–1702. [Google Scholar] [CrossRef]
- Liu, S.P.; Qu, X.S. Construction of nanocomposite film of Dawson-type polyoxometalate and TiO2 nanowires for electrochromic applications. Appl. Surf. Sci. 2017, 412, 189–195. [Google Scholar] [CrossRef]
- Ahmed, I.; Wang, X.X.; Boualili, N.; Xu, H.L.; Farha, R.; Goldmann, M.; Ruhlmann, L. Photocatalytic synthesis of silver dendrites using electrostatic hybrid Films of porphyrin–polyoxometalate. Appl. Catal. A Gen. 2012, 447, 89–99. [Google Scholar] [CrossRef]
- Walls, J.M.; Sagu, J.S.; Wijayantha, K.G. Upul. Microwave synthesised Pd–TiO2 for photocatalytic ammonia production. RSC Adv. 2019, 9, 6387–6394. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Ma, H.Y.; Chen, Y.Y.; Pang, H.J.; Yu, Y. Amperometric detection of nitrite based on Dawson-type vanodotungstophosphate and carbon nanotubes. Anal. Chim. Acta 2013, 792, 35–44. [Google Scholar] [CrossRef]
- Sun, S.B.; Tang, C.J.; Jiang, Y.C.; Wang, D.S.; Chang, X.T.; Lei, Y.H.; Wang, N.N.; Zhu, Y.Q. Flexible and rechargeable electrochromic aluminium-ion battery based on tungsten oxide film electrode. Sol. Energy Mater. Sol. Cells 2020, 207, 110332. [Google Scholar] [CrossRef]
- Cai, G.F.; Chen, J.W.; Xiong, J.Q.; Lee-Sie Eh, A.; Wang, J.X.; Higuchi, M.; Lee, P.S. Molecular level assembly for high-performance flexible electrochromic energy-storage devices. ACS Energy Lett. 2020, 5, 1159–1166. [Google Scholar] [CrossRef]
- Sajitha, S.; Aparna, U.; Deb, B. Ultra-thin manganese dioxide-encrusted vanadium pentoxide nanowire mats for electrochromic energy storage applications. Adv. Mater. Interfaces 2019, 6, 2001928. [Google Scholar] [CrossRef]
- Gu, X.; Liu, Y.B.; Wang, J.X.; Xiao, X.D.; Ca, X.S.; Sheng, G.Z. Synthesis of high-performance electrochromic thin films by a low-cost method. Ceram. Int. 2021, 47, 7837–7844. [Google Scholar]
- Libansky, M.; Zima, J.; Barek, J.; Reznickova, A.; Svorcik, V.; Dejmkova, H. Basic electrochemical properties of sputtered gold film electrodes. Electrochim. Acta 2017, 251, 452–460. [Google Scholar] [CrossRef]
- Shi, Y.D.; Sun, M.J.; Zhang, Y.; Cui, J.W.; Wu, Y.Q.; Ta, H.H.; Liu, J.Q.; Wu, Y.C. Structure modulated amorphous/crystalline WO3 nanoporous arrays with superior electrochromic energy storage performance. Sol. Energy Mater. Sol. Cells 2020, 212, 110579. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Jia, X.T.; Zhu, M.H.; Liu, X.C.; Chao, D.M. Oligoaniline-functionalized polysiloxane/prussian blue composite towards bifunctional electrochromic supercapacitors. New J. Chem. 2020, 44, 8138–8147. [Google Scholar] [CrossRef]
- Guo, Q.F.; Zhao, X.Q.; Li, Z.Y.; Wang, B.Y.; Wang, D.B.; Nie, G.M. High performance multicolor intelligent supercapacitor and its quantitative monitoring of energy storage level by electrochromic parameters. ACS Appl. Energy Mater. 2020, 3, 2727–2736. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Wang, S.; Zhou, S.J.; Xu, H.B.; Zhao, J.P.; Wang, J.; Li, Y. Electrochromic-supercapacitor based on MOF derived hierarchical-porous NiO film. Nanoscale 2020, 12, 8934–8941. [Google Scholar] [CrossRef]
- Shi, Y.D.; Sun, M.J.; Chen, W.J.; Zhang, Y.; Shu, X.; Qin, Y.Q.; Zhang, X.R.; Shen, H.J.; Wu, Y.C. Rational construction of porous amorphous WO3 nanostructures with high electrochromic energy storage performance: Effect of temperature. J. Non-Cryst. Solids 2020, 549, 120337. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Yang, Y.; Chu, D.; Liu, Z.; Zhou, L.; Yu, X.; Qu, X. Vanadium-Substituted Dawson-Type Polyoxometalate–TiO2 Nanowire Composite Film as Advanced Cathode Material for Bifunctional Electrochromic Energy-Storage Devices. Molecules 2022, 27, 4291. https://doi.org/10.3390/molecules27134291
Fu Y, Yang Y, Chu D, Liu Z, Zhou L, Yu X, Qu X. Vanadium-Substituted Dawson-Type Polyoxometalate–TiO2 Nanowire Composite Film as Advanced Cathode Material for Bifunctional Electrochromic Energy-Storage Devices. Molecules. 2022; 27(13):4291. https://doi.org/10.3390/molecules27134291
Chicago/Turabian StyleFu, Yu, Yanyan Yang, Dongxue Chu, Zefeng Liu, Lili Zhou, Xiaoyang Yu, and Xiaoshu Qu. 2022. "Vanadium-Substituted Dawson-Type Polyoxometalate–TiO2 Nanowire Composite Film as Advanced Cathode Material for Bifunctional Electrochromic Energy-Storage Devices" Molecules 27, no. 13: 4291. https://doi.org/10.3390/molecules27134291
APA StyleFu, Y., Yang, Y., Chu, D., Liu, Z., Zhou, L., Yu, X., & Qu, X. (2022). Vanadium-Substituted Dawson-Type Polyoxometalate–TiO2 Nanowire Composite Film as Advanced Cathode Material for Bifunctional Electrochromic Energy-Storage Devices. Molecules, 27(13), 4291. https://doi.org/10.3390/molecules27134291