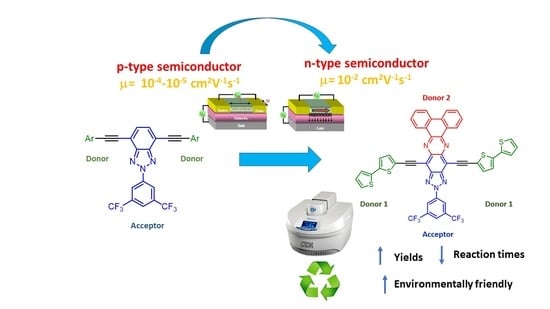
Microwave Irradiation as a Powerful Tool for the Preparation of n-Type Benzotriazole Semiconductors with Applications in Organic Field-Effect Transistors
Abstract
:1. Introduction
2. Results
2.1. Theoretical Calculations
2.2. Synthesis
2.3. Photophysical Data
2.4. Electrical Characterization
3. Materials and Methods
3.1. General Techniques
3.2. Experimental Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gedye, R.; Smith, F.; Westaway, K.; Ali, H.; Baldisera, L.; Laberge, L.; Rousell, J. The use of microwave ovens for rapid organic synthesis. Tetrahedron Lett. 1986, 27, 279–282. [Google Scholar] [CrossRef]
- Giguere, R.J.; Bray, T.L.; Duncan, S.M.; Majetich, G. Application of commercial microwave ovens to organic synthesis. Tetrahedron Lett. 1986, 27, 4945–4948. [Google Scholar] [CrossRef]
- De la Hoz, A.; Loypy, A. Microwaves in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Sanhi, R.; Singh, V. Green Chemistry for Environmental Remediation; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Pollastri, M.P.; Devine, W.G. Microwave Synthesis in Green Techniques for Organic Synthesis and Medicinal Chemistry; Wiley: Chichester, UK, 2012. [Google Scholar]
- Horikoshi, S.; Serpone, N. Microwaves in Nanoparticles Synthesis: Fundamentals and Applications; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Tierney, J.P.; Lidstrom, P. Microwave Assisted Organic Synthesis; Blackwell Publishing Ltd.: Oxford, UK, 2009. [Google Scholar]
- Leadbeater, N.E. Organic Synthesis Using Microwave Heating, in Comprehensive Organic Synthesis, 2nd ed.; Elsevier Ltd.: Oxford, UK, 2014. [Google Scholar]
- Kappe, C.O.; Stadler, A. Microwaves in Organic and Medicinal Chemistry, 2nd ed.; Wiley: Weinheim, Germany, 2012. [Google Scholar]
- Baig, R.B.N.; Varma, R.S. Alternative energy input: Mechanochemical, microwave and ultrasound-assisted organic synthesis. Chem. Soc. Rev. 2012, 41, 1559–1584. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.L.; Tofteng, A.P.; Malik, L.; Jensen, K.J. Microwave heating in solid-phase peptide synthesis. Chem. Soc. Rev. 2012, 41, 1826–1844. [Google Scholar] [CrossRef] [Green Version]
- Takkellapati, S.R. Microwave-assisted Chemical Transformations. Curr. Org. Chem. 2013, 17, 2305–2322. [Google Scholar] [CrossRef]
- Kappe, C.O. How to measure reaction temperature in microwave-heated transformations. Chem. Soc. Rev. 2013, 42, 4977–4990. [Google Scholar] [CrossRef]
- Wu, Z.; Borreto, E.; Medlock, J.; Bonrath, W.; Cravotto, G. Effects of Ultrasound and Microwaves on Selective Reduction: Catalyst Preparation and Reactions. Chem. Cat. Chem. 2014, 6, 2762–2783. [Google Scholar] [CrossRef]
- Stefanidis, G.D.; Munoz, A.N.; Sturm, G.S.J.; Stankiewicz, A. A helicopter view of microwave application to chemical processes: Reactions, separations, and equipment concepts. Rev. Chem. Eng. 2014, 30, 233–259. [Google Scholar] [CrossRef] [Green Version]
- Mingos, D.M.P.; Baghurst, D.R. Tilden Lecture. Applications of microwave dielectric heating effects to synthetic problems in chemistry. Chem. Soc. Rev. 1991, 20, 1–47. [Google Scholar] [CrossRef]
- Shaikh, A. Application of Microwaves in Sustainable Organic Synthesis in Green Chemistry, an Inclusive Approach; Elsevier Ltd.: Oxford, UK, 2018. [Google Scholar]
- Albuquerque, H.M.T.; Pinto, D.C.G.A.; Silva, A.M.S. Microwave Irradiation: Alternative Heating Process for the Synthesis of Biologically Applicable Chromones, Quinolones, and Their Precursors. Molecules 2021, 26, 6293. [Google Scholar] [CrossRef]
- Majhi, S. The Art of Total Synthesis of Bioactive Natural Products via Microwaves. Curr. Org. Chem. 2021, 25, 1047–1069. [Google Scholar] [CrossRef]
- Banik, B.K. Microwave-Induced Organic Reactions toward Biologically Active Molecules. Curr. Med. Chem. 2019, 26, 4492–4494. [Google Scholar] [CrossRef]
- Martina, K.; Tagliapietra, S.; Veselov, V.V.; Cravotto, G. Green Protocols in Heterocycle Syntheses via 1,3-Dipolar Cycloadditions. Front. Chem. 2019, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Floris, B.; Sabuzi, F.; Galloni, P.; Conte, V. The Beneficial Sinergy of MW Irradiation and Ionic Liquids in Catalysis of Organic Reactions. Catalysts 2017, 7, 261. [Google Scholar] [CrossRef] [Green Version]
- Besson, T.; Fruit, C. Recent Developments in Microwave-Assisted Metal-Catalyzed C–H Functionalization of Heteroarenes for Medicinal Chemistry and Material Applications. Synthesis 2016, 48, 3879–3889. [Google Scholar] [CrossRef]
- Komati, S.; Sauvager, A.; Bazureau, J.-P.; Tomasi, S. Efficiency and selectivity of ionic liquids in microwave-assisted extraction of major lichen phenolic compounds: A scalable process with recycling of ionic liquids. Phytochem. Anal. 2021, 32, 592–600. [Google Scholar] [CrossRef]
- Díaz-Ortiz, A.; Díez-Barra, E.; de la Hoz, A.; Prieto, P.; Moreno, A.; Langa, F.; Prangé, T.; Neuman, A. Facial Selectivity in Cycloadditions of a Chiral Ketene Acetal under Microwave Irradiation in Solvent-Free Conditions. Configurational Assignment of the Cycloadducts by NOESY Experiments and Molecular Mechanics Calculations. J. Org. Chem. 1995, 60, 4160–4166. [Google Scholar] [CrossRef]
- Díaz-Ortiz, A.; Carrillo, J.R.; Gómez-Escalonilla, M.J.; de la Hoz, A.; Moreno, A.; Prieto, P. First Diels-Alder Reaction of Pyrazolyl Imines under Microwave Irradiation. Synlett 1998, 10, 1069–1070. [Google Scholar] [CrossRef]
- de la Hoz, A.; Díaz-Ortiz, A.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef]
- Brunetti, F.G.; Herrero, M.A.; Munoz, J.D.; Díaz-Ortiz, A.; Alfonsi, J.; Meneghetti, M.; Prato, M.; Vazquez, E. Microwave-Induced Multiple Functionalization of Carbon Nanotubes. J. Am. Chem. Soc. 2008, 130, 8094–8100. [Google Scholar] [CrossRef]
- de la Hoz, A.; Díaz-Ortiz, A.; Gómez, M.V.; Prieto, P.; Migallón, A.S. Elucidation of Microwave Effects: Methods, Theories, and Predictive Models. In Microwaves in Organic Synthesis, 3rd ed.; Wiley: Weinheim, Germany, 2013. [Google Scholar]
- Prieto, P.; de la Hoz, A.; Díaz-Ortiz, A.; Rodríguez, A.M. Understanding MAOS through computational chemistry. Chem. Soc. Rev. 2017, 46, 431–451. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Ortiz, A.; de la Hoz, A.; Prieto, P. A Critical Overview on the Effect of Microwave Irradiation in Organic Synthesis. Chem. Rec. 2019, 19, 85–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neto, B.A.D.; Carvalho, P.H.P.R.; Correa, J.R. Benzothiadiazole Derivatives as Fluorescence Imaging Probes: Beyond Classical Scaffolds. Acc. Chem. Res. 2015, 48, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Someya, T.; Bao, Z.; Malliaras, G.G. The rise of plastic bioelectronics. Nature 2016, 540, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Pei, Q. Electronic Muscles and Skins: A Review of Soft Sensors and Actuators. Chem. Rev. 2017, 117, 11239–11268. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, W.; Ma, Y.; Wang, H.; Qi, L.; Cao, Y.; Wang, J.; Pei, J. Single Microwire Transistors of Oligoarenes by Direct Solution Process. J. Am. Chem. Soc. 2007, 129, 12386–12387. [Google Scholar] [CrossRef]
- Briseno, A.L.; Mannsfeld, S.C.B.; Reese, C.; Hancook, J.; Xiong, Y.; Jenekhe, S.A.; Bao, Z.; Xia, Y. Perylenediimide Nanowires and Their Use in Fabricating Field-Effect Transistors and Complementary Inverters. Nano Lett. 2007, 7, 2847–2853. [Google Scholar] [CrossRef]
- Jiang, L.; Fu, Y.; Li, H.; Hu, W. Single-Crystalline, Size, and Orientation Controllable Nanowires and Ultralong Microwires of Organic Semiconductor with Strong Photoswitching Property. J. Am. Chem. Soc. 2008, 130, 3937–3941. [Google Scholar] [CrossRef]
- Yang, J.; Yan, D.; Jones, T.S. Molecular Template Growth and Its Applications in Organic Electronics and Optoelectronics. Chem. Rev. 2015, 115, 5570–5603. [Google Scholar] [CrossRef]
- Patel, D.; Fude, F.; Ohnishi, Y.; Abboud, K.; Hirata, S.H.; Schanze, K.S.; Reynolds, J.R. It Takes More Than an Imine: The Role of the Central Atom on the Electron-Accepting Ability of Benzotriazole and Benzothiadiazole Oligomers. J. Am. Chem. Soc. 2012, 134, 2599–2612. [Google Scholar] [CrossRef]
- Dong, Y.; Cal, W.; Wang, M.; Li, Q.; Yilg, L.; Huang, F.; Cao, Y. [1,2,5]Thiadiazolo 3,4-f]benzotriazole based narrow band gap conjugated polymers with photocurrent response up to 1.1 μm. Org. Electron. 2013, 14, 2459–2467. [Google Scholar] [CrossRef]
- Dong, Y.; Cai, W.; Hu, X.; Zhong, C.; Huang, F.; Cao, Y. Synthesis of novel narrow-band-gap copolymers based on [1,2,5]thiadiazolo[3,4-f] benzotriazole and their application in bulk-heterojunction photovoltaic devices. Polymer 2012, 53, 1465–1471. [Google Scholar] [CrossRef]
- Torres-Moya, I.; Benitez-Martín, C.; Donoso, B.; Tardio, C.; Martín, R.; Carrillo, J.R.; Díaz-Ortiz, A.; Nájera, F.; Prieto, P.; Pérez-Inestrosa, E. Extended Alkenyl and Alkynyl Benzotriazoles with Enhanced Two-Photon Absorption Properties as a Promising Alternative to Benzothiadiazoles. Chem. Eur. J. 2019, 25, 15572–15579. [Google Scholar] [CrossRef] [PubMed]
- Torres-Moya, I.; Saikia, B.; Prieto, P.; Carrillo, J.R.; Steed, B.J.W. High thermal stability, pH responsive organogelsof 2H-benzo[d]1,2,3-triazole derivatives as pharmaceutical crystallization media. CrystEngComm 2019, 21, 2135–2142. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, R.; Martinez-Tomé, M.J.; Kahveci, Z.; Torres-Moya, I.; Falco, A.; Mallavia, R.; Reyes, C. Synthesis and Characterization of a Novel Green Cationic Polyfluorene and Its Potential Use as a Fluorescent Membrane Probe. Polymers 2018, 10, 938. [Google Scholar] [CrossRef] [Green Version]
- Torres-Moya, I.; Arrechea-Marcos, I.; Tardío, C.; Carrillo, J.R.; Díaz-Ortiz, A.; López Navarrete, J.T.; Ruiz Delgado, M.C.; Prieto, P.; Ponce, R. D–A–D 2H-benzo[d][1,2,3]triazole derivatives as p-type semiconductors in organic field-effect transistors. RSC Adv. 2018, 8, 21879–21888. [Google Scholar] [CrossRef] [Green Version]
- Bredas, J.L.; Beljonne, D.; Coropceanu, V.; Cornil, J. Charge-transfer and energy-transfer processes in π-conjugated oligomers and Polymers: A molecular picture. J. Chem. Rev. 2004, 104, 4971–5004. [Google Scholar] [CrossRef]
- Ruiz Delgado, M.C.; Kim, E.G.; da Silva, D.A.; Bredas, J.L. Tuning the Charge-Transport Parameters of Perylene Diimide Single Crystals via End and/or Core Functionalization: A Density Functional Theory Investigation. J. Am. Chem. Soc. 2010, 132, 3375–3387. [Google Scholar] [CrossRef]
- Wettach, H.; Pasker, F.; Höger, S. 2-Aryl-2H-benzotriazoles as Building Blocks for New Low-Bandgap Poly(arylene-ethynylene). Macromolecules 2008, 41, 9513–9515. [Google Scholar] [CrossRef]
- Torres, I.; Díaz-Ortiz, A.; Sánchez, L.; Orduña, J.; Blesa, M.J.; Carrillo, J.R.; Prieto, P. Tunable emission in aggregated T-Shaped 2H-Benzo[d][1,2,3]triazoles with waveguide behaviour. Dyes Pigm. 2017, 142, 212–225. [Google Scholar] [CrossRef] [Green Version]
- Kwon, P.-S.; Kim, J.-K.; Kwon, T.W.; Kim, Y.-H.; Chung, S.-K. Microwave Irradiated Acetylation and Nitration of Aromatic Compounds. Bull. Korean. Chem. Soc. 1997, 18, 10. [Google Scholar] [CrossRef]
- Moran, M.J.; Martina, K.; Stefanidis, G.D.; Jordens, J.; Gerven, T.B.; Goovaerts, V.; Manzoli, M.; Groffils, C.; Cravotto, G. Glycerol: An Optimal Hydrogen Source for Microwave-Promoted Cu-Catalyzed Transfer Hydrogenation of Nitrobenzene to Aniline. Front Chem. 2020, 29, 8–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velázquez, D.G.; Luque, R.; Gutiérrez Ravelo, A. Microwave-Assisted Synthesis and Properties of Novel Hexaazatrinaphthylene Dendritic Scaffolds. Molecules 2020, 25, 5038. [Google Scholar] [CrossRef] [PubMed]
- Pastor, M.J.; Torres, I.; Cebrián, C.; Carrillo, J.R.; Díaz-Ortiz, A.; Matesanz, E.; Buendía, J.; García, F.; Barberá, J.; Prieto, P.; et al. 4-Aryl-3,5-bis(arylethynyl)aryl-4H-1,2,4-triazoles: Multitasking Skeleton as a Self-Assembling Unit. Chem. Eur. J. 2015, 21, 1795–1802. [Google Scholar] [CrossRef] [PubMed]
- Torres, I.; Carrillo, J.R.; Díaz-Ortiz, A.; Martín, R.; Gómez, M.V.; Stegemann, L.; Strassert, C.A.; Orduna, J.; Buendía, J.; Greciano, E.E.; et al. Self-assembly of T-shape 2H-benzo[d][1,2,3]-triazoles. Optical waveguide and photophysical properties. RSC Adv. 2016, 6, 36544–36553. [Google Scholar] [CrossRef]
- Size, S.M.; Lee, M.K. Semiconductor devices: Physics and Technology, 3rd ed.; Wiley: New York, NY, USA, 2012; ISBN 978-0-470-53794-7. [Google Scholar]
- Ponce, R.; Facchetti, A.; Marks, T. High-k Organic, Inorganic, and Hybrid Dielectrics for Low-Voltage Organic Field-Effect Transistors. J. Chem. Rev. 2010, 110, 205–239. [Google Scholar] [CrossRef]
- Jo, Y.; Gyu Oh, J.; Kim, C.; Kyu An, T.; Jang, J.; Lee, J. Synthetic strategy for thienothiophene-benzotriazole-based polymers with high backbone planarity and solubility for field-effect transistor applications. J. Ind. Eng. Chem. 2020, 86, 150–157. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Molecular Modeling and Synthesis of Ethyl Benzyl Carbamates as Possible Ixodicide Activity; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Becke, A.D. Densityfunctional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5650. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Kim, F.S.; Ren, G.; Hollenbeck, E.C.; Subramaniyan, S.; Jenekhe, S.A. Tetraazabenzodifluoranthene diimides: Building blocks for solution-processable n-type organic semiconductors. Angew. Chem. Int. Ed. 2013, 52, 5513–5517. [Google Scholar] [CrossRef]







| Product | Conventional Conditions | Microwave Irradiation | ||
|---|---|---|---|---|
| Reaction Time | Yield (%) | Reaction Time | Yield (%) | |
| 4 | 24 h | 77 | 20 min | 94 |
| 6 | 12 h | 58 | 30 min | 78 |
| 7 | 7 h | 55 | 10 min | 80 |
| 8 | 12 h | 60 | 10 min | 92 |
| 9 | 24 h | 94 | 30 min | 100 |
| 11 | 15 h | 88 | 30 min | 90 |
| 1 | 24 h | 10 | 20 min | 71 |
| Overall yield | 1% | 35% | ||
| Total reaction time | 118 h | 2 h 30 min | ||
| Compound | λabs (nm) | λem (nm) | Φ |
|---|---|---|---|
| 1 | 426, 588, 635 | 673 | 0.58 |
| Substrate Functionalization/Thermal Treatment | μe (cm2V–1s–1) | VT (V) | ION/IOFF |
|---|---|---|---|
| HMDS/90 °C | 1.4 × 10–2 | 46 | 7.26 × 102 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Moya, I.; Harbuzaru, A.; Donoso, B.; Prieto, P.; Ponce Ortiz, R.; Díaz-Ortiz, Á. Microwave Irradiation as a Powerful Tool for the Preparation of n-Type Benzotriazole Semiconductors with Applications in Organic Field-Effect Transistors. Molecules 2022, 27, 4340. https://doi.org/10.3390/molecules27144340
Torres-Moya I, Harbuzaru A, Donoso B, Prieto P, Ponce Ortiz R, Díaz-Ortiz Á. Microwave Irradiation as a Powerful Tool for the Preparation of n-Type Benzotriazole Semiconductors with Applications in Organic Field-Effect Transistors. Molecules. 2022; 27(14):4340. https://doi.org/10.3390/molecules27144340
Chicago/Turabian StyleTorres-Moya, Iván, Alexandra Harbuzaru, Beatriz Donoso, Pilar Prieto, Rocío Ponce Ortiz, and Ángel Díaz-Ortiz. 2022. "Microwave Irradiation as a Powerful Tool for the Preparation of n-Type Benzotriazole Semiconductors with Applications in Organic Field-Effect Transistors" Molecules 27, no. 14: 4340. https://doi.org/10.3390/molecules27144340
APA StyleTorres-Moya, I., Harbuzaru, A., Donoso, B., Prieto, P., Ponce Ortiz, R., & Díaz-Ortiz, Á. (2022). Microwave Irradiation as a Powerful Tool for the Preparation of n-Type Benzotriazole Semiconductors with Applications in Organic Field-Effect Transistors. Molecules, 27(14), 4340. https://doi.org/10.3390/molecules27144340