Succinimido–Ferrocidiphenol Complexed with Cyclodextrins Inhibits Glioblastoma Tumor Growth In Vitro and In Vivo without Noticeable Adverse Toxicity
Abstract
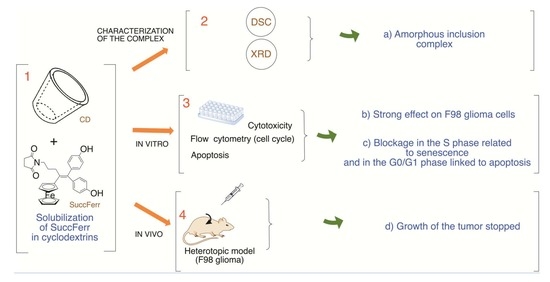
:1. Introduction
2. Results
2.1. Physicochemical Characterization of the Freeze-Dried SuccFerr Complex
2.2. Pharmaceutical Properties
2.2.1. Release Profile of SuccFerr:RAMEßCD Complex
2.2.2. Anticancer Activity
2.2.3. Hemolytic Activity
2.3. Animal Study
2.3.1. Acute Toxicity Study of SuccFerr:RAMEβCD Administered Intravenously or Subcutaneously
2.3.2. Body and Organ Weight: Maximum Tolerated Dose (MTD)
2.3.3. Hematological and Biochemical Parameters
2.3.4. In Vivo Evaluation of SuccFerr:RAMEβCD Anticancer Activity in a Murine Glioblastoma Model
2.3.5. Histological Analyses
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis and Quantification of Succinimido–Ferrocidiphenol (SuccFerr)
4.2.1. Chemical Synthesis of SuccFerr
4.2.2. Quantification of SuccFerr
4.3. Solid Phase Analysis of Succ:RAMEβCD
4.3.1. X-ray Diffraction (XRD)
4.3.2. Differential Scanning Calorimeter (DSC)
4.4. Pharmaceutical Properties of the SuccFerr:RAMEßCD Complex
4.4.1. Dissolution and Diffusivity across a Dialysis Membrane
4.4.2. Cell Culture Experiments
4.4.3. In Vitro Cytotoxicity on Glioblastoma U87 and F98 Cells in Culture
4.4.4. Cell Cycle Analysis
4.4.5. Cell Apoptosis Assay
4.4.6. Hemolytic Activity
4.5. Animal Studies
4.5.1. Animal and Anesthesia
4.5.2. Acute Intravenous or Subcutaneously Toxicity Study in Rats
4.5.3. Maximum Tolerated Dose (MTD): Sub-Acute Intraperitoneal or Subcutaneous Toxicity Study in Rats
4.5.4. Measurement of Hematological and Biochemical Parameters in Rats
4.5.5. In Vivo Antitumor Efficacy
4.5.6. Histological Analyses
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jaouen, G.; Beck, W.; Mc Glinchey, M.J. A Novel Field of Research: Bioorganometallic Chemistry, Origins, and Founding Principles. In Bioorganometallics; Jaouen, G., Ed.; Wiley-VCH: Weinheim, Germany, 2005; pp. 1–37. [Google Scholar]
- Hartinger, C.G.; Dyson, P.J. Bioorganometallic chemistry—from teaching paradigms to medicinal applications. Chem. Soc. Rev. 2009, 38, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Hillard, E.A.; Jaouen, G. Bioorganometallics: Future Trends in Drug Discovery, Analytical Chemistry, and Catalysis. Organometallics 2011, 30, 20–27. [Google Scholar] [CrossRef]
- Reedijk, J. Platinum Anticancer Coordination Compounds: Study of DNA Binding Inspires New Drug Design. Eur. J. Inorg. Chem. 2009, 2009, 1303–1312. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Z. Targeting and delivery of platinum-based anticancer drugs. Chem. Soc. Rev. 2013, 42, 202–224. [Google Scholar] [CrossRef]
- Barry, N.P.E.; Sadler, P.J. Exploration of the medical periodic table: Towards new targets. Chem. Comm. 2013, 49, 5106–5131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaouen, G.; Vessieres, A.; Top, S. Ferrocifen type anti cancer drugs. Chem. Soc. Rev. 2015, 44, 8802–8817. [Google Scholar] [CrossRef] [Green Version]
- Murray, B.S.; Dyson, P.J. Recent progress in the development of organometallics for the treatment of cancer. Curr. Opin. Chem. Biol. 2019, 56, 28–34. [Google Scholar] [CrossRef]
- Vessieres, A. Iron Compounds as Anticancer Agents. In Metal-based Anticancer Agents; The Royal Society of Chemistry: Cambridge, UK, 2019; pp. 62–90. [Google Scholar]
- Skoupilova, H.; Bartosik, M.; Sommerova, L.; Pinkas, J.; Vaculovic, T.; Kanicky, V.; Karban, J.; Hrstka, R. Ferrocenes as new anticancer drug candidates: Determination of the mechanism of action. Eur. J. Pharmacol. 2020, 867, 172825. [Google Scholar] [CrossRef]
- Fouda, M.F.R.; Abd-Elzaher, M.M.; Abdelsamaia, R.A.; Labib, A.A. On the medicinal chemistry of ferrocene. Appl. Organomet. Chem. 2007, 21, 613–625. [Google Scholar] [CrossRef]
- Tonolo, F.; Salmain, M.; Scalcon, V.; Top, S.; Pigeon, P.; Folda, A.; Caron, B.; McGlinchey, M.J.; Toillon, R.A.; Bindoli, A.; et al. Small Structural Differences between Two Ferrocenyl Diphenols Determine Large Discrepancies of Reactivity and Biological Effects. Chem. Med. Chem. 2019, 14, 1717–1726. [Google Scholar] [CrossRef]
- Wang, Y.; Pigeon, P.; Top, S.; Sanz|García, J.; Troufflard, C.; Ciofini, I.; McGlinchey, M.J.; Jaouen, G. Atypical Lone Pair–π Interaction with Quinone Methides in a Series of Imido-Ferrociphenol Anticancer Drug Candidates. Angew. Chem. Int. Ed. Engl. 2019, 58, 8421–8425. [Google Scholar] [CrossRef] [PubMed]
- Vessières, A.; Wang, Y.; McGlinchey, M.J.; Jaouen, G. Multifaceted chemical behaviour of metallocene (M = Fe, Os) quinone methides. Their contribution to biology. Coord. Chem. Rev. 2021, 430, 213658. [Google Scholar] [CrossRef]
- Sharma, B.; Kumar, V. Has Ferrocene Really Delivered Its Role in Accentuating the Bioactivity of Organic Scaffolds? J. Med.Chem. 2021, 64, 16865–16921. [Google Scholar] [CrossRef]
- Bjørklund, G.; Zou, L.; Wang, J.; Chasapis, C.T.; Peana, M. Thioredoxin reductase as a pharmacological target. Pharmacol. Res. 2021, 174, 105854. [Google Scholar] [CrossRef]
- Richard, M.A.; Hamels, D.; Pigeon, P.; Top, S.; Dansette, P.M.; Lee, H.Z.; Vessieres, A.; Mansuy, D.; Jaouen, G. Oxidative metabolism of ferrocene analogues of tamoxifen: Characterization and antiproliferative activities of the metabolites. Chem. Med. Chem. 2015, 10, 981–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Dansette, P.M.; Pigeon, P.; Top, S.; McGlinchey, M.J.; Mansuy, D.; Jaouen, G. A new generation of ferrociphenols leads to a great diversity of reactive metabolites, and exhibits remarkable antiproliferative properties. Chem. Sci. 2018, 9, 70–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pautu, V.; Lepeltier, E.; Mellinger, A.; Riou, J.; Debuigne, A.; Jérôme, C.; Clere, N.; Passirani, C. pH-Responsive Lipid Nanocapsules: A Promising Strategy for Improved Resistant Melanoma Cell Internalization. Cancers 2021, 13, 2028. [Google Scholar] [CrossRef]
- Idlas, P.; Lepeltier, E.; Jaouen, G.; Passirani, C. Ferrocifen Loaded Lipid Nanocapsules: A Promising Anticancer Medication against Multidrug Resistant Tumors. Cancers 2021, 13, 2291. [Google Scholar] [CrossRef]
- Pigeon, P.; Najlaoui, F.; McGlinchey, M.J.; Sanz García, J.; Jaouen, G.; Gibaud, S. Manipulation of a large database containing information about cyclodextrin-ferrociphenol supramolecular assemblies. submitted.
- Vessières, A.; Quissac, E.; Lemaire, N.; Alentorn, A.; Domeracka, P.; Pigeon, P.; Sanson, M.; Idbaih, A.; Verreault, M. Heterogeneity of Response to Iron-Based Metallodrugs in Glioblastoma Is Associated with Differences in Chemical Structures and Driven by FAS Expression Dynamics and Transcriptomic Subtypes. Int. J. Mol. Sci. 2021, 22, 10404. [Google Scholar] [CrossRef]
- Li, J.; Kataoka, K. Chemo-physical Strategies to Advance the in Vivo Functionality of Targeted Nanomedicine: The Next Generation. J. Am. Chem. Soc. 2020, 143, 538–559. [Google Scholar] [CrossRef]
- Becherirat, S.; Lanhers, M.-C.; Socha, M.; Yemloul, M.; Astier, A.; Loboda, C.; Aniceto, N.; Gibaud, S. The antitumor effects of an arsthinol–cyclodextrin complex in a heterotopic mouse model of glioma. Eur. J. Pharm. Biopharm. 2013, 85, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Trapani, G.; Latrofa, A.; Franco, M.; Pantaleo, M.R.; Sanna, E.; Massa, F.; Tuveri, F.; Liso, G. Complexation of zolpidem with 2-hydroxypropyl-beta-, methyl-beta-, and 2-hydroxypropyl-gamma-cyclodextrin: Effect on aqueous solubility, dissolution rate, and ataxic activity in rat. J. Pharm. Sci. 2000, 89, 1443–1451. [Google Scholar] [CrossRef]
- Najlaoui, F.; Pigeon, P.; Abdelkafi, Z.; Leclerc, S.; Durand, P.; Ayeb, M.E.; Marrakchi, N.; Rhouma, A.; Jaouen, G.; Gibaud, S. Phthalimido-ferrocidiphenol cyclodextrin complexes: Characterization and anticancer activity. Int. J. Pharm. 2015, 491, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Jodál, I.; Nánási, P.; Szejtli, J. Investigation of the Hemolytic Effect of the Cyclodextrin Derivatives. In Advances in Inclusion Science; Huber, O., Szejtli, J., Eds.; Springer: Dordrecht, The Netherlands, 1988; Volume 5, pp. 421–425. [Google Scholar]
- Vessières, A.; Top, S.; Pigeon, P.; Hillard, E.; Boubeker, L.; Spera, D.; Jaouen, G. Modification of the Estrogenic Properties of Diphenols by the Incorporation of Ferrocene. Generation of Antiproliferative Effects in Vitro. J. Med. Chem. 2005, 48, 3937–3940. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, A.; Marsaud, V.; Bouclier, C.; Top, S.; Vessieres, A.; Pigeon, P.; Gref, R.; Legrand, P.; Jaouen, G.; Renoir, J.-M. Nanoparticles loaded with ferrocenyl tamoxifen derivatives for breast cancer treatment. Int. J. Pharm. 2008, 347, 128–135. [Google Scholar] [CrossRef]
- Najlaoui, F.; Pigeon, P.; Aroui, S.; Pezet, M.; Sancey, L.; Marrakchi, N.; Rhouma, A.; Jaouen, G.; De Waard, M.; Busser, B.; et al. Anticancer properties of lipid and poly(epsilon-caprolactone) nanocapsules loaded with ferrocenyl-tamoxifen derivatives. J. Pharm. Pharmacol. 2018, 70, 1474–1484. [Google Scholar] [CrossRef]
- Topin-Ruiz, S.; Mellinger, A.; Lepeltier, E.; Bourreau, C.; Fouillet, J.; Riou, J.; Jaouen, G.; Martin, L.; Passirani, C.; Clere, N. p722 ferrocifen loaded lipid nanocapsules improve survival of murine xenografted-melanoma via a potentiation of apoptosis and an activation of CD8+ T lymphocytes. Int. J. Pharm. 2021, 593, 120111. [Google Scholar] [CrossRef]
- Laine, A.L.; Clavreul, A.; Rousseau, A.; Tetaud, C.; Vessieres, A.; Garcion, E.; Jaouen, G.; Aubert, L.; Guilbert, M.; Benoit, J.P.; et al. Inhibition of ectopic glioma tumor growth by a potent ferrocenyl drug loaded into stealth lipid nanocapsules. Nanomedicine 2014, 10, 1667–1677. [Google Scholar] [CrossRef]
- Bruyère, C.; Mathieu, V.; Vessières, A.; Pigeon, P.; Top, S.; Jaouen, G.; Kiss, R. Ferrocifen derivatives that induce senescence in cancer cells: Selected examples. J. Inorg. Biochem. 2014, 141, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Olson, H.; Betton, G.; Robinson, D.; Thomas, K.; Monro, A.; Kolaja, G.; Lilly, P.; Sanders, J.; Sipes, G.; Bracken, W.; et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 2000, 32, 56–67. [Google Scholar] [CrossRef]
- Petterino, C.; Argentino-Storino, A. Clinical chemistry and haematology historical data in control Sprague-Dawley rats from pre-clinical toxicity studies. Exp. Toxicol. Pathol. 2006, 57, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Son, C.G.; Han, S.H.; Cho, J.H.; Shin, J.W.; Cho, C.H.; Lee, Y.W.; Cho, C.K. Induction of hemopoiesis by Saenghyuldab, a mixture of Ginseng Radix, Paeoniae Radix Alba, and Hominis Placenta extracts. Acta Pharmacol. Sin. 2003, 24, 120–126. [Google Scholar] [PubMed]
- Aniagu, S.O.; Nwinyi, F.C.; Akumka, D.D.; Ajoku, G.A.; Dzarma, S.; Izebe, K.S.; Ditse, M.; Nwaneri, P.E.C.; Wambebe, C.; Gamaniel, K. Toxicity studies in rats fed nature cure bitters. Afr. J. Biotechnol. 2005, 4, 72–78. [Google Scholar]
- Hassan, S.W.; Ladan, M.J.; Dogondaji, R.A.; Umar, R.A.; Bilbis, L.S.; Hassan, L.G.; Ebbo, A.A.; Matazu, I.K. Phytochemical and toxicological studies of aqueous leaves extracts of Erythrophleum africanum. Pak. J. Biol. Sci. 2007, 10, 3815–3821. [Google Scholar] [PubMed] [Green Version]
- Rhiouani, H.; El-Hilaly, J.; Israili, Z.H.; Lyoussi, B. Acute and sub-chronic toxicity of an aqueous extract of the leaves of Herniaria glabra in rodents. J. Ethnopharmacol. 2008, 118, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Pigeon, P.; Wang, Y.; Top, S.; Najlaoui, F.; Garcia Alvarez, M.C.; Bignon, J.; McGlinchey, M.J.; Jaouen, G. A New Series of Succinimido-ferrociphenols and Related Heterocyclic Species Induce Strong Antiproliferative Effects, Especially against Ovarian Cancer Cells Resistant to Cisplatin. J. Med. Chem. 2017, 60, 8358–8368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higuchi, T.; Connors, K. Phase-solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Hansen, M.; Nielsen, S. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Meth. 1989, 119, 203–210. [Google Scholar] [CrossRef]
- Malagoli, D. A full-length protocol to test hemolytic activity of palytoxin on human erythrocytes. ISJ Invertebr. Surviv. J. 2007, 4, 92–94. [Google Scholar]
- Taiwe, G.S.; Bum, E.N.; Talla, E.; Dimo, T.; Weiss, N.; Sidiki, N.; Dawe, A.; Moto, F.C.; Dzeufiet, P.D.; De Waard, M. Antipyretic and antinociceptive effects of Nauclea latifolia root decoction and possible mechanisms of action. Pharm. Biol. 2011, 49, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Litchfield, J.T., Jr.; Wilcoxon, F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar] [PubMed]
- Tan, P.V.; Mezui, C.; Enow-Orock, G.; Njikam, N.; Dimo, T.; Bitolog, P. Teratogenic effects, acute and sub chronic toxicity of the leaf aqueous extract of Ocimum suave Wild (Lamiaceae) in rats. J. Ethnopharmacol. 2008, 115, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A.; Saraf, S. Cyclodextrin based novel drug delivery systems. J. Incl. Phenom. Macrocycl. Chem. 2008, 62, 23–42. [Google Scholar] [CrossRef]
- Levin, V.A. Relationship of octanol/water partition coefficient and molecular weight to rat brain capillary permeability. J. Med. Chem. 1980, 23, 682–684. [Google Scholar] [CrossRef] [PubMed]
- Hirohashi, T.; Terasaki, T.; Shigetoshi, M.; Sugiyama, Y. In vivo and in vitro evidence for nonrestricted transport of 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein tetraacetoxymethyl ester at the blood-brain barrier. J. Pharmacol. Exp. Ther. 1997, 280, 813–819. [Google Scholar]
- Pardridge, W.M. CNS Drug Design Based on Principles of Blood-Brain Barrier Transport. J. Neurochem. 1998, 70, 1781–1792. [Google Scholar] [CrossRef]













| Treatments | Dose (mg/kg) | D/T | Mortality Latency (h) | Toxic Symptoms |
|---|---|---|---|---|
| Physiological saline solution | - | 0/5 | - | None |
| SuccFerr:RAMEβCD | 25 | 0/5 | - | None |
| SuccFerr:RAMEβCD | 50 | 0/5 | - | None |
| SuccFerr:RAMEβCD | 100 | 0/5 | - | None |
| SuccFerr:RAMEβCD | 200 | 2/5 | >24, <36 | None |
| SuccFerr:RAMEβCD | 400 | 3/5 | >36, <48 | Hypoactivity, piloerection, salivation, asthenia |
| SuccFerr:RAMEβCD | 800 | 3/5 | >24, <36 | Hypoactivity, piloerection, salivation, syncope |
| SuccFerr:RAMEβCD | 1600 | 5/5 | >24, <36 | Hypoactivity, asthenia, anorexia, salivation, syncope |
| SuccFerr:RAMEβCD | 3200 | 5/5 | >24, <36 | Hypoactivity, asthenia, anorexia, salivation, syncope |
| SuccFerr:RAMEβCD | 6400 | 5/5 | >24, <36 | Hypoactivity, asthenia, anorexia, salivation, syncope |
| Parameter | Vehicle | Treatments (mg/kg) | ||
|---|---|---|---|---|
| 0.5 | 1 | 1.5 | ||
| Heart | 0.35 ± 0.01 | 0.35 ± 0.01 | 0.35 ± 0.01 | 0.35 ± 0.01 |
| Liver | 3.35 ± 0.01 | 3.38 ± 0.02 | 3.82 ± 0.04 | 3.84 ± 0.05 |
| Lung | 0.88 ± 0.04 | 0.87 ± 0.04 | 0.86 ± 0.03 | 0.85 ± 0.03 |
| Spleen | 0.72 ± 0.02 | 0.72 ± 0.01 | 0.76 ± 0.01 | 0.76 ± 0.01 |
| Kidney | 0.62 ± 0.01 | 0.66 ± 0.01 | 0.67 ± 0.01 | 0.68 ± 0.01 |
| Parameters | Treatments (mg/kg) | |||
|---|---|---|---|---|
| Vehicle | 0.5 | 1 | 1.5 | |
| RBC (×106 µL−1) | 6.62 ± 0.13 | 6.35 ± 0.13 | 6.36 ± 0.17 | 5.32 ± 0.11 |
| Hemoglobin (g/dL) | 11.43 ± 0.12 | 11.58 ± 0.15 | 11.37 ± 0.92 | 9.35 ± 0.33 |
| Hematocrit (%) | 37.02 ± 0.16 | 38.19 ± 1.04 | 41.59 ± 0.79 | 37.22 ± 0.04 |
| MCV (fL) | 58.28 ± 0.35 | 58.76 ± 0.54 | 58.50 ± 0.53 | 57.68 ± 1.03 |
| MCH (pg) | 17.49 ± 0.15 | 17.49 ± 0.21 | 17.60 ± 0.19 | 17.60 ± 0.19 |
| MCHC (g/dL) | 29.56 ± 0.16 | 30.39 ± 0.22 | 30.51 ± 0.22 | 30.59 ± 0.14 |
| Platelets (×103 µL−1) | 466.20 ± 7.44 | 456.60 ± 14.08 | 473.60 ± 9.28 | 421.40 ± 7.12 |
| WBC (×103 µL−1) | 13.49 ± 0.21 | 15.46 ± 0.45 | 11.32 ± 0.24 | 9.76 ± 0.47 |
| Neutrophils (%) | 22.66 ± 0.34 | 24.75 ± 1.75 | 20.62 ± 0.46 | 20.47 ± 0.24 |
| Eosinophils (%) | 2.54 ± 0.14 | 3.25 ± 0.18 | 2.55 ± 0.17 | 2.75 ± 0.08 |
| Basophils (%) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Lymphocytes (%) | 68.88 ± 0.29 | 67.18 ± 1.12 | 65.14 ± 0.87 | 66.24 ± 0.26 |
| Monocytes (%) | 5.68 ± 0.18 | 6.34 ± 0.14 | 5.48 ± 0.25 | 5.18 ± 0.25 |
| Parameters | Treatments (mg/kg) | |||
|---|---|---|---|---|
| Vehicle | 0.5 | 1 | 1.5 | |
| Glu (g/L) | 0.58 ± 0.03 | 0.62 ± 0.05 | 0.62 ± 0.01 | 0.58 ± 0.03 |
| AST (IU/L) | 217.24 ± 1.55 | 237.44 ± 8.13 | 269.30 ± 8.28 * | 269.96 ± 18.85 * |
| ALT (IU/L) | 51.72 ± 0.15 | 55.28 ± 0.98 | 55.16 ± 0.38 | 66.12 ± 1.18 * |
| ALP (IU/L) | 338.14 ± 0.54 | 337.78 ± 0.61 | 323.32 ± 3.68 | 301.64 ± 5.76 * |
| TP (g/L) | 71.92 ± 0.61 | 68.24 ± 0.59 | 72.74 ± 0.76 | 73.78 ± 0.62 |
| ALB (g/L) | 21.28 ± 0.15 | 23.80 ± 0.17 | 24.2 ± 0.13 | 24.12 ± 0.19 |
| TB (mg/L) | 6.56 ± 0.43 | 7.24 ± 0.43 | 7.68 ± 0.66 | 7.86 ± 0.80 |
| CB (mg/L) | 3.76 ± 0.25 | 4.44 ± 0.37 | 4.68 ± 0.18 | 5.66 ± 0.37 |
| TC (g/L) | 0.60 ± 0.02 | 0.61 ± 0.01 | 0.61 ± 0.01 | 0.62 ± 0.04 |
| LDL (g/L) | 0.19 ± 0.01 | 0.19 ± 0.01 | 0.19 ± 0.02 | 0.19 ± 0.01 |
| HDL (g/L) | 0.538 ± 0.020 | 0.53 ± 0.02 | 0.53 ± 0.02 | 0.54 ± 0.02 |
| TG (g/L) | 1.43 ± 0.13 | 1.63 ± 0.09 | 1.75 ± 0.06 | 1.63 ± 0.03 |
| Parameters | Treatments (mg/kg) | |||
|---|---|---|---|---|
| Vehicle | 0.5 | 1 | 1.5 | |
| Creatinine (mg/L) | 7.32 ± 0.26 | 8.94 ± 0.31 | 8.88 ± 0.19 | 7.35 ± 0.27 |
| Urea (mg/L) | 0.422 ± 0.010 | 0.39 ± 0.01 | 0.39 ± 0.05 | 0.425 ± 0.020 |
| Uric acid (mg/L) | 46.92 ± 0.52 | 45.88 ± 0.73 | 43.66 ± 1.19 | 46.90 ± 0.50 |
| Na+ (mEquiv./L) | 134.72 ± 0.51 | 134.24 ± 1.29 | 131.06 ± 2.59 | 134.76 ± 0.50 |
| Cl− (mEquiv./L) | 85.64 ± 1.03 | 85.74 ± 1.74 | 86.72 ± 1.08 | 84.46 ± 1.51 |
| K+ (mEquiv./L) | 3.44 ± 0.21 | 3.64 ± 0.18 | 3.36 ± 0.23 | 3.42 ± 0.25 |
| Ca2+ (mg/L) | 77.94 ± 0.47 | 78.52 ± 0.42 | 69.22 ± 1.14 | 67.04 ± 1.51 |
| Mg2+ (mg/L) | 17.87 ± 0.16 | 19.28 ± 0.47 | 18.19 ± 0.64 | 19.55 ± 0.67 |
| Inorganic phosphorus (mg/L) | 33.08 ± 1.37 | 30.52 ± 1.32 | 29.58 ± 0.65 | 29.18 ± 1.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najlaoui, F.; Busser, B.; Taïwe, G.S.; Pigeon, P.; Sturm, N.; Giovannini, D.; Marrakchi, N.; Rhouma, A.; Jaouen, G.; Gibaud, S.; et al. Succinimido–Ferrocidiphenol Complexed with Cyclodextrins Inhibits Glioblastoma Tumor Growth In Vitro and In Vivo without Noticeable Adverse Toxicity. Molecules 2022, 27, 4651. https://doi.org/10.3390/molecules27144651
Najlaoui F, Busser B, Taïwe GS, Pigeon P, Sturm N, Giovannini D, Marrakchi N, Rhouma A, Jaouen G, Gibaud S, et al. Succinimido–Ferrocidiphenol Complexed with Cyclodextrins Inhibits Glioblastoma Tumor Growth In Vitro and In Vivo without Noticeable Adverse Toxicity. Molecules. 2022; 27(14):4651. https://doi.org/10.3390/molecules27144651
Chicago/Turabian StyleNajlaoui, Feten, Benoit Busser, Germain Sotoing Taïwe, Pascal Pigeon, Nathalie Sturm, Diane Giovannini, Naziha Marrakchi, Ali Rhouma, Gérard Jaouen, Stéphane Gibaud, and et al. 2022. "Succinimido–Ferrocidiphenol Complexed with Cyclodextrins Inhibits Glioblastoma Tumor Growth In Vitro and In Vivo without Noticeable Adverse Toxicity" Molecules 27, no. 14: 4651. https://doi.org/10.3390/molecules27144651
APA StyleNajlaoui, F., Busser, B., Taïwe, G. S., Pigeon, P., Sturm, N., Giovannini, D., Marrakchi, N., Rhouma, A., Jaouen, G., Gibaud, S., & De Waard, M. (2022). Succinimido–Ferrocidiphenol Complexed with Cyclodextrins Inhibits Glioblastoma Tumor Growth In Vitro and In Vivo without Noticeable Adverse Toxicity. Molecules, 27(14), 4651. https://doi.org/10.3390/molecules27144651