Piperidine-Iodine as Efficient Dual Catalyst for the One-Pot, Three-Component Synthesis of Coumarin-3-Carboxamides
Abstract
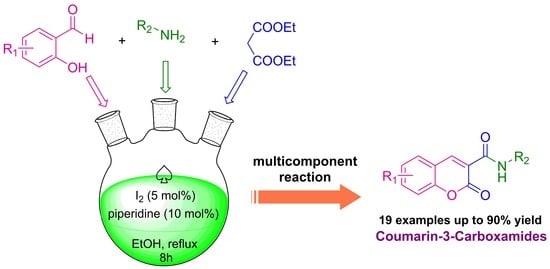
:1. Introduction
2. Results
3. Materials and Methods
3.1. General Information
3.2. General Procedures and Compound Characterization Data for Coumarin-3-carboxamides 3a–n and 7a–e
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bienaymé, H.; Hulme, C.; Oddon, G.; Schmitt, P. Maximizing Synthetic Efficiency: Multi-Component Transformations Lead the Way. Chem. Eur. J. 2000, 6, 3321–3329. [Google Scholar] [CrossRef]
- Ruijter, E.; Scheffelaar, R.; Orru, R.V.A. Multicomponent Reaction Design in the Quest for Molecular Complexity and Diversity. Angew. Chem. Int. Ed. 2011, 50, 6234–6246. [Google Scholar] [CrossRef] [PubMed]
- Zhi, S.; Ma, X.; Zhang, W. Consecutive multicomponent reactions for the synthesis of complex molecules. Org. Biomol. Chem. 2019, 17, 7632–7650. [Google Scholar] [CrossRef]
- Dömling, A.; Wang, W.; Wang, K. Chemistry and Biology Of Multicomponent Reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Dömling, A. Multicomponent Reactions in Medicinal Chemistry. In Multicomponent Reactions towards Heterocycles: Concepts and Applications; Van der Eycken, E.V., Sharma, U.K., Eds.; John Wiley and Sons: Weinheim, Germany, 2021; pp. 91–137. [Google Scholar]
- Murray, R.D.H. Coumarins. Nat. Prod. Rep. 1995, 12, 477–505. [Google Scholar] [CrossRef]
- Peng, X.M.; Damu, L.V.; Zhou, C.H. Current developments of coumarin compounds in medicinal chemistry. Curr. Pharm. Des. 2013, 19, 3884–3930. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.S.; Gumireddy, K.; Mallireddigari, M.R.; Cosenza, S.C.; Venkatapuram, P.; Bell, S.C.; Reddy, E.P.; Reddy, M.R. Novel coumarin-3-(N-aryl)carboxamides arrest breast cancer cell growth by inhibiting ErbB-2 and ERK1. Bioorg. Med. Chem. 2005, 13, 3141–3147. [Google Scholar] [CrossRef] [PubMed]
- Kempen, I.; Hemmer, M.; Counerotte, S.; Pochet, L.; de Tullio, P.; Foidart, J.M.; Blacher, S.; Noe¨l, A.; Frankenne, F.; Pirott, B. 6- Substituted 2-oxo-2H-1-benzopyran-3-carboxylic acid derivatives in a new approach of the treatment of cancer cell invasion and metastasis. Eur. J. Med. Chem. 2008, 43, 2735–2750. [Google Scholar] [CrossRef]
- Melagraki, G.; Afantitis, A.; Igglessi-Markopoulou, O.; Detsi, A.; Koufaki, M.; Kontogiorgis, C.; Hadjipavlou-Litina, D.J. Synthesis and evaluation of the antioxidant and anti-inflammatory activity of novel coumarin-3-aminoamides and their alpha-lipoic acid adducts. Eur. J. Med. Chem. 2009, 44, 3020–3026. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Q.-W.; Shi, Y.; Song, Z.-G.; Jin, Y.-H.; Liu, Z.-Q. Design and synthesis of coumarin-3-acylamino derivatives to scavenge radicals and to protect DNA. Eur. J. Med. Chem. 2014, 84, 1–7. [Google Scholar] [CrossRef]
- E Bylov, I.; Vasylyev, M.V.; Bilokin, Y.V. Synthesis and anti-inflammatory activity of N-substituted 2-oxo-2H-1-benzopyran-3-carboxamides and their 2-iminoanalogues. Eur. J. Med. Chem. 1999, 34, 997–1001. [Google Scholar] [CrossRef]
- Robert, S.; Bertolla, C.; Masereel, B.; Dogné, J.-M.; Pochet, L. Novel 3-Carboxamide-coumarins as Potent and Selective FXIIa Inhibitors. J. Med. Chem. 2008, 51, 3077–3080. [Google Scholar] [CrossRef] [PubMed]
- Edraki, N.; Firuzi, O.; Foroumadi, A.; Miri, R.; Madadkar-Sobhani, A.; Khoshneviszadeh, M.; Shafiee, A. Phenylimino-2 H -chromen-3-carboxamide derivatives as novel small molecule inhibitors of β-secretase (BACE1). Bioorg. Med. Chem. 2013, 21, 2396–2412. [Google Scholar] [CrossRef] [PubMed]
- Iraji, A.; Firuzi, O.; Khoshneviszadeh, M.; Tavakkoli, M.; Mahdavi, M.; Nadri, H.; Edraki, N.; Miri, R. Multifunctional iminochromene-2H-carboxamide derivatives containing different aminomethylene triazole with BACE1 inhibitory, neuroprotective and metal chelating properties targeting Alzheimer’s disease. Eur. J. Med. Chem. 2017, 141, 690–702. [Google Scholar] [CrossRef]
- Pan, Z.-X.; He, X.; Chen, Y.-Y.; Tang, W.-J.; Shi, J.-B.; Tang, Y.-L.; Song, B.-A.; Li, J.; Liu, X.-H. New 2H-chromene-3-carboxamide derivatives: Design, synthesis and use as inhibitors of hMAO. Eur. J. Med. Chem. 2014, 80, 278–284. [Google Scholar] [CrossRef]
- Kamauchi, H.; Noji, M.; Kinoshita, K.; Takanami, T.; Koyama, K. Coumarins with an unprecedented tetracyclic skeleton and coumarin dimers from chemically engineered extracts of a marine-derived fungus. Tetrahedron 2018, 74, 2846–2856. [Google Scholar] [CrossRef]
- Endo, S.; Matsunaga, T.; Kuwata, K.; Zhao, H.-T.; El-Kabbani, O.; Kitade, Y.; Hara, A. Chromene-3-carboxamide derivatives discov ered from virtual screening as potent inhibitors of the tumor maker, AKR1B10. Bioorg. Med. Chem. 2010, 18, 2485–2490. [Google Scholar] [CrossRef]
- Qian, B.; Váradi, L.; Trinchi, A.; Reichman, S.M.; Bao, L.; Lan, M.; Wei, G.; Cole, I.S. The Design and Synthesis of Fluorescent Coumarin Derivatives and Their Study for Cu2+ Sensing with an Application for Aqueous Soil Extracts. Molecules 2019, 24, 3569. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Dou, W.; Qin, W.; Liu, W. A new coumarin-based chemosensor for Fe3+ in water. Inorg. Chem. Commun. 2009, 12, 116–118. [Google Scholar] [CrossRef]
- Yoshihara, T.; Yamaguchi, Y.; Hosaka, M.; Takeuchi, T.; Tobita, S. Ratiometric Molecular Sensor for Monitoring Oxygen Levels in Living Cells. Angew. Chem. Int. Ed. 2012, 51, 4148–4151. [Google Scholar] [CrossRef]
- Chimenti, F.; Secci, D.; Bolasco, A.; Chimenti, P.; Bizzarri, B.; Granese, A.; Carradori, S.; Yáñez, M.; Orallo, F.; Ortuso, F.; et al. Synthesis, Molecular Modeling, and Selective Inhibitory Activity against Human Monoamine Oxidases of 3-Carboxamido-7-Substituted Coumarins. J. Med. Chem. 2009, 52, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Proenca, F.; Costa, M. A simple and eco-friendly approach for the synthesis of 2-imino and 2-oxo-2H-chromene-3-carboxamides. Green Chem. 2008, 10, 995–998. [Google Scholar] [CrossRef]
- Sepay, N.; Guha, C.; Kool, A.; Mallik, A.K. An efficient three-component synthesis of coumarin-3-carbamides by use of Ni–NiO nanoparticles as magnetically separable catalyst. RSC Adv. 2015, 5, 70718–70725. [Google Scholar] [CrossRef]
- Payra, S.; Saha, A.; Banerjee, S. Magnetically Recoverable Fe3O4 Nanoparticles for the One-Pot Synthesis of coumarin-3-carboxamide derivatives in aqueous ethanol. Chem. Sel. 2018, 3, 7535–7540. [Google Scholar]
- Gupta, M.; Kumar, P.; Bahadur, V.; Kumar, K.; Parmar, V.S.; Singh, B.K. Metal-Free, Regioselective, Dehydrogenative Cross-Coupling between Formamides/Aldehydes and Coumarins by C–H Functionalization. Eur. J. Org. Chem. 2018, 2018, 896–900. [Google Scholar] [CrossRef]
- Wang, L.; Ding, S.; Shen, H.; Wang, Y.; Hao, S.; Yin, G.; Qiu, J.; Lin, b.; Wu, Z.; Zhao, M. Generation of Coumarin-3-Carboxamides From Coumarin-3-Carboxylic Acids and Tetraalkylthiuram Disulfides Catalyzed by Copper Salts. Asian J. Org. Chem. 2021, 10, 2544–2548. [Google Scholar] [CrossRef]
- Hideo, T.; Shinpei, I. Synthetic use of molecular iodine for organic synthesis. Synlett 2006, 14, 2159–2175. [Google Scholar]
- Jereb, M.; Vražič, D.; Zupan, M. Iodine-catalyzed transformation of molecules containing oxygen functional groups. Tetrahedron 2011, 67, 1355–1387. [Google Scholar] [CrossRef]
- Parvatkar, P.T.; Parameswaran, P.S.; Tilve, S.G. Recent Developments in the Synthesis of Five- and Six-Membered Heterocycles Using Molecular Iodine. Chem. Eur. J. 2012, 18, 5460–5489. [Google Scholar] [CrossRef]
- Ren, Y.-M.; Cai, C.; Yang, R.-C. Molecular iodine-catalyzed multicomponent reactions: An efficient catalyst for organic synthesis. RSC Adv. 2013, 3, 7182–7204. [Google Scholar] [CrossRef]
- Alizadeh, A.; Ghanbaripour, R.; Zhu, L.G. An efficient approach to the synthesis of coumarin-bearing 2, 3-dihydro-4 (1H)-quinazolinone derivatives using a piperidine and molecular iodine dual-catalyst system. Synlett 2014, 25, 1596–1600. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Palnati, G.R.; Singh, L.R.; Upadhyay, A.; Avula, S.R.; Kumar, A.; Kant, R. Molecular iodine catalysed one-pot synthesis of chromeno [4, 3-b] quinolin-6-ones under microwave irradiation. Green Chem. 2015, 17, 3766–3770. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, W.; Qian, M.; Zhao, T.; Yang, L.; Zhu, C. Iodine-promoted three-component reaction for the synthesis of spirooxindoles. Tetrahedron 2018, 74, 955–961. [Google Scholar] [CrossRef]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvent. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Fonseca, A.; Gaspar, A.; Matos, M.J.; Gomes, L.R.; Low, J.N.; Uriarte, E.; Borges, F. Structural elucidation of a series of 6-methyl-3-carboxamidocoumarins. Org. Magn. Reson. 2017, 55, 373–378. [Google Scholar] [CrossRef]
- Mohammed, H.B. Conversion of 3-carbethoxy-4-methyl coumarin derivatives into several new annelated coumarin derivatives. Chin. J. Chem. 2003, 21, 1219–1223. [Google Scholar] [CrossRef]
- Hu, S.; Wang, J.; Huang, G.; Zhu, K.; Chen, F. Organocatalytic Asymmetric Domino Oxa-Michael–Mannich-[1, 3]-Amino Rearrangement Reaction of N-Tosylsalicylimines to α, β-Unsaturated Aldehydes by Diarylprolinol Silyl Ethers. J. Org. Chem. 2020, 85, 4011–4018. [Google Scholar] [CrossRef]
- Breugst, M.; von der Heiden, D. Mechanisms in iodine catalysis. Chem. Eur. J. 2018, 24, 9187–9199. [Google Scholar] [CrossRef]
- Chen, G.F.; Jia, H.M.; Zhang, L.Y.; Hu, J.; Chen, B.H.; Song, Y.L.; Li, J.T.; Bai, G.Y. A highly selective fluorescent sensor for Fe3+ ion based on coumarin derivatives. Res. Chem. Intermed. 2013, 39, 4081–4090. [Google Scholar] [CrossRef]
- Areias, F.; Costa, M.; Castro, M.; Brea, J.; Gregori-Puigjané, E.; Proença, F.; Mestres, J.; Loza, M.I. New chromene scaffolds for adenosine A2A receptors: Synthesis, pharmacology and structure–activity relationships. Eur. J. Med. Chem. 2012, 54, 303–310. [Google Scholar] [CrossRef]
- Santos-Contreras, R.J.; Martínez-Martínez, F.J.; Mancilla-Margalli, N.A.; Peraza-Campos, A.L.; Morín-Sánchez, L.M.; García-Báez, E.V.; Padilla-Martínez, I.I. Competition between OH⋯O and multiple halogen–dipole interactions on the formation of intramolecular three-centred hydrogen bond in 3-acyl coumarins. CrystEngComm 2009, 11, 1451–1461. [Google Scholar] [CrossRef]
- Shi, J.; Lu, W.; Chen, J.; Sun, L.; Yang, S.; Zhou, M.; Xu, L.; Ma, Y.; Yu, L. Synthesis, antiproliferative activities, and DNA binding of coumarin-3-formamido derivatives. Arch. Pharm. 2021, 354, 2000236. [Google Scholar] [CrossRef] [PubMed]
- Ghanei-Nasab, S.; Khoobi, M.; Hadizadeh, F.; Marjani, A.; Moradi, A.; Nadri, H.; Emami, S.; Foroumadi, A.; Shafiee, A. Synthesis and anticholinesterase activity of coumarin-3-carboxamides bearing tryptamine moiety. Eur. J. Med. Chem. 2016, 121, 40–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Teng, P.; Zhang, Y.-L.; Xu, Z.-J.; Zhang, M.-Z.; Zhang, W.-H. Design, synthesis and antifungal activity evaluation of coumarin-3-carboxamide derivatives. Fitoterapia 2018, 127, 387–395. [Google Scholar] [CrossRef]
- Jang, Y.J.; Syu, S.E.; Chen, Y.J.; Yang, M.C.; Lin, W. Syntheses of furo[3, 4-c] coumarins and related furyl coumarin derivatives via intramolecular Wittig reactions. Org. Biomol. Chem. 2012, 10, 843–847. [Google Scholar] [CrossRef]







 | |||||
|---|---|---|---|---|---|
| Entry | Solvent | Base (mol%) | Iodine (mol%) | Ratio b (3a:4a:5) | Yield 3a c (%) |
| 1 | EtOH | piperidine 10% | 10 | 100:0:0 | 79 |
| 2 | MeOH | piperidine 10% | 10 | 100:0:0 | 50 |
| 3 | i-PrOH | piperidine 10% | 10 | 100:0:0 | 38 |
| 4 | t-BuOH | piperidine 10% | 10 | 100:0:0 | 37 |
| 5 | H2O | piperidine 10% | 10 | 35:55:10 | 14 |
| 6 | CH2Cl2 | piperidine 10% | 10 | 28:2:70 | 8 |
| 7 | MeCN | piperidine 10% | 10 | 75:0:25 | 32 |
| 8 | EtOAc | piperidine 10% | 10 | 51:14:35 | 26 |
| 9 | THF | piperidine 10% | 10 | 66:24:10 | 17 |
| 10 | DMF | piperidine 10% | 10 | 21:53:26 | 10 |
| 11 | Solvent free | piperidine 10% | 10 | 100:0:0 | 36 |
| 12 | EtOH | Et3N 10% | 10 | 100:0:0 | 40 |
| 13 | EtOH | DBU 10% | 10 | 100:0:0 | 49 |
| 14 | EtOH | L-Proline 10% | 10 | 100:0:0 | 35 |
| 15 | EtOH | piperidine 10% | 20 | 100:0:0 | 75 |
| 16 | EtOH | piperidine 10% | 15 | 100:0:0 | 79 |
| 17 | EtOH | piperidine 10% | 5 | 100:0:0 | 85 |
| 18 | EtOH | piperidine 10% | 1 | 100:0:0 | 60 |
| 19 | EtOH | piperidine 5% | 5 | 100:0:0 | 72 |
| 20 | EtOH | - | 5 | 100:0:0 | 63 |
| 21 | EtOH | piperidine 10% | - | 100:0:0 | 66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velasco, M.; Romero-Ceronio, N.; Torralba, R.; Hernández Abreu, O.; Vilchis-Reyes, M.A.; Alarcón-Matus, E.; Ramos-Rivera, E.M.; Aparicio, D.M.; Jiménez, J.; Aguilar García, E.; et al. Piperidine-Iodine as Efficient Dual Catalyst for the One-Pot, Three-Component Synthesis of Coumarin-3-Carboxamides. Molecules 2022, 27, 4659. https://doi.org/10.3390/molecules27144659
Velasco M, Romero-Ceronio N, Torralba R, Hernández Abreu O, Vilchis-Reyes MA, Alarcón-Matus E, Ramos-Rivera EM, Aparicio DM, Jiménez J, Aguilar García E, et al. Piperidine-Iodine as Efficient Dual Catalyst for the One-Pot, Three-Component Synthesis of Coumarin-3-Carboxamides. Molecules. 2022; 27(14):4659. https://doi.org/10.3390/molecules27144659
Chicago/Turabian StyleVelasco, Manuel, Nancy Romero-Ceronio, Rosalía Torralba, Oswaldo Hernández Abreu, Miguel A. Vilchis-Reyes, Erika Alarcón-Matus, Erika M. Ramos-Rivera, David M. Aparicio, Jacqueline Jiménez, Eric Aguilar García, and et al. 2022. "Piperidine-Iodine as Efficient Dual Catalyst for the One-Pot, Three-Component Synthesis of Coumarin-3-Carboxamides" Molecules 27, no. 14: 4659. https://doi.org/10.3390/molecules27144659
APA StyleVelasco, M., Romero-Ceronio, N., Torralba, R., Hernández Abreu, O., Vilchis-Reyes, M. A., Alarcón-Matus, E., Ramos-Rivera, E. M., Aparicio, D. M., Jiménez, J., Aguilar García, E., Cruz Cruz, D., Villegas Gómez, C., & Alvarado, C. (2022). Piperidine-Iodine as Efficient Dual Catalyst for the One-Pot, Three-Component Synthesis of Coumarin-3-Carboxamides. Molecules, 27(14), 4659. https://doi.org/10.3390/molecules27144659