Cytotoxic and Antioxidant Activities of Imine Analogs of Trans-Resveratrol towards Murine Neuronal N2a Cells
Abstract
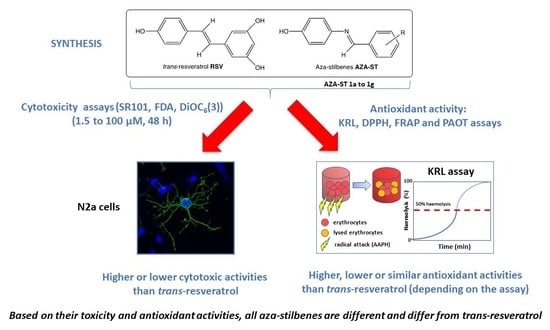
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Cytotoxicity
3. Conclusions
4. Materials and Methods
4.1. Chemistry
4.2. Cell Culture and Treatments
4.3. Measurement of Cell Density: Sulforhodamine 101 (SR101) Assay
4.4. Measurement of Esterase Activity: Fluorescein Diacetate (FDA) Assay
4.5. Measurement of Transmembrane Mitochondrial Potential (ΔΨm): 3,3′-Dihexyloxacarbocyanine IODIDE (DiOC6(3)) Assay
4.6. Measurement of Antioxidant Activity with the KRL (Kit Radicaux Libres) Assay
4.7. Measurement of Antioxidant Activity with the DPPH (2,2′-Diphenyl-1-Picrylhydrazyl Radical) Assay
4.8. Measurement of Antioxidant Activity with the Ferric Reducing Antioxidant (FRAP) Assay
4.9. Measurement of Antioxidant Activity with the PAOT Liquid® Technology Assay
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities and synthesis. Angew. Chem. 2011, 50, 596–621. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, M.; Durazzo, A.; Lombardi-Boccia, G.; Souto, E.B.; Cecchini, F.; Santini, A. Wine plolyphenols and health: Quantitative research literature analysis. Appl. Sci. 2021, 11, 4762. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, X.; Wang, B.; Yin, G.; Wang, J.; Wang, T.; Bi, K. Based on multi-activity integrated strategy to screening, characterization and quantification of bioactive compounds from red wine. Molecules 2021, 26, 6750. [Google Scholar] [CrossRef]
- Colica, C.; Milanovic, M.; Milié, N.; Aiello, V.; De Lorenzo, A.; Abenavoli, L. A systematic review on natural antioxidant properties of resveratrol. Nat. Prod. Commun. 2018, 13, 1195–1203. [Google Scholar] [CrossRef]
- Yamine, A.; Namsi, A.; Vervandier-Fasseur, D.; Mackrill, J.J.; Lizard, G.; Latruffe, N. Polyphenols of the mediterranean diet and their metabolites in the prevention of colorectal cancer. Molecules 2021, 26, 3483. [Google Scholar] [CrossRef] [PubMed]
- Pezzuto, J.M. Resveratrol: Twenty years of growth, development and controversy. Biomol. Ther. 2019, 27, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Walle, T. Bioavailability of resveratrol. Ann. N.Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Cardile, V.; Chillemi, R.; Lombardo, L.; Sciuto, S.; Spatafora, C.; Tringali, C. Antiproliferative activity of methylated analogues of E- and Z-resveratrol. Z. Naturforsch. C 2007, 62, 189–195. [Google Scholar] [CrossRef]
- Liu, Q.; Kim, C.T.; Jo, Y.H.; Kim, S.B.; Hwang, B.Y.; Lee, M.K. Synthesis and biological evaluation of resveratrol derivatives as melanogenesis inhibitors. Molecules 2015, 20, 16933–16945. [Google Scholar] [CrossRef]
- Nawaz, W.; Zhou, Z.; Deng, S.; Ma, X.; Ma, X.; Li, C.; Shu, X. Therapeutic versatility of resveratrol derivatives. Nutrients 2017, 9, 1188. [Google Scholar] [CrossRef]
- Chalal, M.; Vervandier-Fasseur, D.; Meunier, P.; Cattey, H.; Hierso, J.C. Syntheses of polyfunctionalized resveratrol derivatives using Wittig and Heck protocols. Tetrahedron 2012, 68, 3899–3907. [Google Scholar] [CrossRef]
- Chalal, M.; Klinguer, A.; Echairi, A.; Meunier, P.; Vervandier-Fasseur, D.; Adrian, M. Antimicrobial activity of resveratrol analogues. Molecules 2014, 19, 7679–7688. [Google Scholar] [CrossRef] [PubMed]
- Chalal, M.; Delmas, D.; Meunier, P.; Latruffe, N.; Vervandier-Fasseur, D. Inhibition of cancer derived cell lines proliferation by newly synthesized hydroxylated stilbenes and ferrocenyl-stilbene analogs. Comparison with resveratrol. Molecules 2014, 19, 7850–7868. [Google Scholar] [CrossRef] [PubMed]
- Latruffe, N.; Vervandier-Fasseur, D. Strategic syntheses of vine and wine resveratrol derivatives to explore their effects on cell functions and dysfunctions. Diseases 2018, 6, 110. [Google Scholar] [CrossRef]
- Belluti, F.; Fontana, G.; Dal Bo, L.; Carenini, N.; Giommarelli, C.; Zunino, F. Design, synthesis and anticancer activities of stilbene-coumarin hybrid compounds: Identification of novel proapopoptic agents. Bioorg. Med. Chem. 2010, 18, 3543–3550. [Google Scholar] [CrossRef]
- Bellina, F.; Guazzelli, N.; Lessi, M.; Manzini, C. Imidazole analogues of resveratrol: Synthesis and cancer cell growth evaluation. Tetrahedron 2015, 71, 2298–2305. [Google Scholar] [CrossRef]
- Li, C.; Xu, X.; Wang, X.J.; Pan, Y. Imine resveratrol analogues: Molecular design, Nrf2 activation and SAR analysis. PLoS ONE 2014, 9, e101455. [Google Scholar]
- Bae, S.J.; Ha, Y.M.; Kim, J.A.; Park, J.Y.; Ha, T.K.; Park, D.; Chun, P.; Park, N.H.; Moon, H.R.; Chung, H.Y. A novel synthesized tyrosinase inhibitor: (E)-2-((2,4-dihydrophenyl)diazinyl)phenyl-4-methylbenzenesulfonate as an azo-resveratrol analog. Biosci. Biotechnol. Biochem. 2013, 77, 65–72. [Google Scholar] [CrossRef]
- Lizard, G.; Latruffe, N.; Vervandier-Fasseur, D. Aza- and Azo-stilbenes: Bio-isosteric analogs of resveratrol. Molecules 2020, 25, 605. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, B.; Pan, Y.; Liang, H.; Yi, X.; Wang, H. Antioxidant activities and transition metal ion chelating studies of some hydroxyl Schiff base derivatives. Med. Chem. Res. 2012, 21, 1341–1346. [Google Scholar] [CrossRef]
- Siddiqui, A.; Dandawate, P.; Rub, R.; Padhye, S.; Aphale, S.; Moghe, A.; Jagyasi, A.; Swamy, K.V.; Singh, B.; Chatterjee, A.; et al. Novel aza-resveratrol analogs: Synthesis, characterization and anti-cancer activity against breast cancer cell lines. Bioorg. Med. Chem. Lett. 2013, 23, 635–640. [Google Scholar] [CrossRef]
- Ronghe, A.; Chatterjee, A.; Singh, B.; Dandawate, P.; Murphy, L.; Bhat, N.K.; Padhye, S.; Bhat, H.K. Differential regulation of estrogen receptors α and β by 4-(E)-{(4-hydroxyphenylimino)-methylbenzene-1,2-diol}, a novel resveratrol analog. J. Steroid Biochem. Mol. Biol. 2014, 144, 500–512. [Google Scholar] [PubMed]
- Tanaka, K.; Shiraishi, R. Clean and efficient condensation reactions of aldehydes and amines in a water suspension medium. Green Chem. 2000, 2, 272–273. [Google Scholar] [CrossRef]
- Lu, J.; Li, C.; Chai, Y.F.; Yang, D.Y.; Sun, C.R. The anti-oxidant effect of imine resveratrol analogues. Bioorg. Med. Chem. Lett. 2012, 22, 5744–5747. [Google Scholar] [CrossRef] [PubMed]
- Kotora, P.; Sersen, F.; Filo, J.; Loos, D.; Gregan, J.; Gregan, F. The scavenging of DPPH, galvinoxyl and ABTS radicals by imine analogs of resveratrol. Molecules 2016, 21, 127. [Google Scholar] [CrossRef]
- Yammine, A.; Zarrouk, A.; Nury, T.; Vejux, A.; Latruffe, N.; Vervandier-Fasseur, D.; Samadi, M.; Mackrill, J.J.; Greige-Gerges, H.; Auezova, L.; et al. Prevention by Dietary Polyphenols (Resveratrol, Quercetin, Apigenin) Against 7-Ketocholesterol-Induced Oxiapoptophagy in Neuronal N2a Cells: Potential Interest for the Treatment of Neurodegenerative and Age-Related Diseases. Cells 2020, 9, 2346. [Google Scholar] [CrossRef]
- Zarrouk, A.; Martine, L.; Grégoire, S.; Nury, T.; Meddeb, W.; Camus, E.; Badreddine, A.; Durand, P.; Namsi, A.; Yammine, A.; et al. Profile of Fatty Acids, Tocopherols, Phytosterols and Polyphenols in Mediterranean Oils (Argan Oils, Olive Oils, Milk Thistle Seed Oils and Nigella Seed Oil) and Evaluation of their Antioxidant and Cytoprotective Activities. Curr. Pharm. Des. 2019, 25, 1791–1805. [Google Scholar] [CrossRef]
- Alashi, A.M.; Taiwo, K.A.; Oyedele, D.J.; Adebooye, O.C.; Aluko, R.E. Polyphenol composition and antioxidant properties of vegetable leaf-fortified bread. J. Food Biochem. 2019, 43, e12625. [Google Scholar] [CrossRef]
- Dudonne, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar]
- Pincemail, J.; Kaci, M.-M.; Kevers, C.; Tabart, J.; Elle, R.E.; Meziane, S. PAOT-Liquid® Technology: An Easy Electrochemical Method for Evaluating Antioxidant Capacity of Wines. Diseases 2019, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Tanski, J.; Villegas-Estrada, A.; Rossi, M. Structural basis for antioxidant activity of trans-resveratrol: Ab initio calculations and crystal and molecular structure. J. Agric. Food Chem. 2004, 52, 7279–7285. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Caruso, F.; Opazo, C.; Salciccioli, J. Crystal and molecular structure of piceatannol: Scavenging features of resveratrol and piceatannol on hydroxyl and peroxyl radicals docking with transthyretin. J. Agric. Food Chem. 2008, 62, 10557–10566. [Google Scholar] [CrossRef] [PubMed]
- Gaenko, A.V.; Devarajan, A.; Gagliardi, L.; Lindh, R.; Orlandi, G. Ab initio DFT study of Z-E isomerization pathways of N-benzylideneaniline. Theor. Chem. Acc. 2007, 118, 271–279. [Google Scholar] [CrossRef]
- Namsi, A.; Nury, T.; Hamdouni, H.; Yammine, A.; Vejux, A.; Vervandier-Fasseur, D.; Latruffe, N.; Masmoudi-Kouki, O.; Lizard, G. Induction of Neuronal Differentiation of Murine N2a Cells by Two Polyphenols Present in the Mediterranean Diet Mimicking Neurotrophins Activities: Resveratrol and Apigenin. Diseases 2018, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Yammine, A.; Nury, T.; Vejux, A.; Latruffe, N.; Vervandier-Fasseur, D.; Samadi, M.; Greige-Gerges, H.; Auezova, L.; Lizard, G. Prevention of 7-Ketocholesterol-Induced Overproduction of Reactive Oxygen Species, Mitochondrial Dysfunction and Cell Death with Major Nutrients (Polyphenols, ω3 and ω9 Unsaturated Fatty Acids) of the Mediterranean Diet on N2a Neuronal Cells. Molecules 2020, 25, 2296. [Google Scholar] [CrossRef] [PubMed]
- Lakey-Beitia, J.; Burillo, A.M.; La Penna, G.; Hegde, M.L.; Rao, K.S. Polyphenols as Potential Metal Chelation Compounds Against Alzheimer’s Disease. J. Alzheimers Dis. 2021, 82, S335–S357. [Google Scholar] [CrossRef]
- Uddin, M.S.; Mamun, A.A.; Rahman, M.M.; Jeandet, P.; Alexiou, A.; Behl, T.; Sarwar, M.S.; Sobarzo-Sánchez, E.; Ashraf, G.M.; Sayed, A.; et al. Natural Products for Neurodegeneration: Regulating Neurotrophic Signals. Oxid. Med. Cell Longev. 2021, 2021, 8820406. [Google Scholar] [CrossRef]
- Kaminski, J.; Lançon, A.; Aires, V.; Limagne, E.; Tili, E.; Michaille, J.J.; Latruffe, N. Resveratrol initiates differentiation of mouse skeletal muscle-derived C2C12 myoblasts. Biochem. Pharmacol. 2012, 84, 1251–1259. [Google Scholar] [CrossRef]
- Cho, M.R.; Lee, S.; Song, S.K. A Review of Sarcopenia Pathophysiology, Diagnosis, Treatment and Future Direction. J. Korean Med. Sci. 2022, 37, e146. [Google Scholar] [CrossRef]
- Namsi, A.; Nury, T.; Khan, A.S.; Leprince, J.; Vaudry, D.; Caccia, C.; Leoni, V.; Atanasov, A.G.; Tonon, M.C.; Masmoudi-Kouki, O.; et al. Octadecaneuropeptide (ODN) Induces N2a Cells Differentiation through a PKA/PLC/PKC/MEK/ERK-Dependent Pathway: Incidence on Peroxisome, Mitochondria, and Lipid Profiles. Molecules 2019, 24, 3310. [Google Scholar] [CrossRef] [PubMed]
- Ragot, K.; Mackrill, J.J.; Zarrouk, A.; Nury, T.; Aires, V.; Jacquin, A.; Athias, A.; Pais de Barros, J.P.; Vejux, A.; Riedinger, J.M.; et al. Absence of correlation between oxysterol accumulation in lipid raft microdomains, calcium increase, and apoptosis induction on 158N murine oligodendrocytes. Biochem. Pharmacol. 2013, 86, 67–79. [Google Scholar] [CrossRef] [PubMed]









| Molecular Formula | Molecular Weight (g/mol) | IC50 µM (SR101) | IC50 µM (FDA) | IC50 µM (DiOC6(3)) | |
|---|---|---|---|---|---|
| Resveratrol | C14H12O3 | 228.24 | ~12.5 | 6.25 < IC50 < 12.5 | 100 |
| Aza-Stilbene 1a | C13H11NO2 | 213.228 | 25 < IC50 < 50 | 12.5 | 50 < IC50 < 100 |
| Aza-Stilbene 1b | C13H11NO2 | 213.228 | 12.5 | 12.5 < IC50 < 25 | 50 < IC50 < 100 |
| Aza-Stilbene 1c | C13H11NO2 | 213.228 | 25 | 12.5 < IC50 < 25 | 50 < IC50 < 100 |
| Aza-Stilbene 1d | C13H11NO3 | 229.228 | 25 | 6.25 | 50 < IC50 < 100 |
| Aza-Stilbene 1e | C13H10NOBr | 227.254 | 25 < IC50 < 50 | 6.25 < IC50 < 12.5 | 50 < IC50 < 100 |
| Aza-Stilbene 1f | C13H10NOBr | 276.11 | 25 < IC50 < 50 | 12.5 | 50 < IC50 < 100 |
| Aza-Stilbene1g | C13H10NOBr | 276.11 | 6.25 | ~12.5 | 50 < IC50 < 100 |
| Antioxidant Activities of Resveratrol and Aza-Stilbenes 1a–1g Evaluated with Different Assays | ||||
|---|---|---|---|---|
| Compounds | KRL (20 µM) | DPPH (25 µM) | FRAP (25 µM) | PAOT Score (25 µM) |
| Resveratrol | 72.81 ± 1.10 | 32.76 ± 6.40 | 10.41 ± 0.05 | 19.39 ± 1.06 |
| Aza-stilbene 1a | 129.74 ± 3.92 | 35.07 ± 1.75 | 10.52 ± 0.05 | 42.65 ± 0.78 |
| Aza-stilbene 1b | 141.29 ± 4.65 | 38.84 ± 1.90 | 10.02 ± 0.04 | 34.11 ± 0.18 |
| Aza-stilbene 1c | 128.38 ± 2.03 | 5.51 ± 2.25 | 12.32 ± 0.06 | 13.34 ± 0.54 |
| Aza-stilbene 1d | 154.63 ± 3.50 | 47.84 ± 2.35 | 11.05 ± 0.07 | 56.49 ± 0.59 |
| Aza-stilbene 1e | 116.06 ± 4.40 | 48.55 ± 2.40 | 10.34 ± 0.05 | ND |
| Aza-stilbene 1f | 123.78 ± 5.16 | 53.69 ± 2.65 | 10.41 ± 0.05 | ND |
| Aza-stilbene 1g | 125.76 ± 3.39 | 17.23 ± 0.85 | 10.18 ± 0.04 | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ksila, M.; Vejux, A.; Prost-Camus, E.; Durand, P.; Ghzaiel, I.; Nury, T.; Duprey, D.; Meziane, S.; Masmoudi-Kouki, O.; Latruffe, N.; et al. Cytotoxic and Antioxidant Activities of Imine Analogs of Trans-Resveratrol towards Murine Neuronal N2a Cells. Molecules 2022, 27, 4713. https://doi.org/10.3390/molecules27154713
Ksila M, Vejux A, Prost-Camus E, Durand P, Ghzaiel I, Nury T, Duprey D, Meziane S, Masmoudi-Kouki O, Latruffe N, et al. Cytotoxic and Antioxidant Activities of Imine Analogs of Trans-Resveratrol towards Murine Neuronal N2a Cells. Molecules. 2022; 27(15):4713. https://doi.org/10.3390/molecules27154713
Chicago/Turabian StyleKsila, Mohamed, Anne Vejux, Emmanuelle Prost-Camus, Philippe Durand, Imen Ghzaiel, Thomas Nury, Dorian Duprey, Smail Meziane, Olfa Masmoudi-Kouki, Norbert Latruffe, and et al. 2022. "Cytotoxic and Antioxidant Activities of Imine Analogs of Trans-Resveratrol towards Murine Neuronal N2a Cells" Molecules 27, no. 15: 4713. https://doi.org/10.3390/molecules27154713
APA StyleKsila, M., Vejux, A., Prost-Camus, E., Durand, P., Ghzaiel, I., Nury, T., Duprey, D., Meziane, S., Masmoudi-Kouki, O., Latruffe, N., Ghrairi, T., Prost, M., Lizard, G., & Vervandier-Fasseur, D. (2022). Cytotoxic and Antioxidant Activities of Imine Analogs of Trans-Resveratrol towards Murine Neuronal N2a Cells. Molecules, 27(15), 4713. https://doi.org/10.3390/molecules27154713