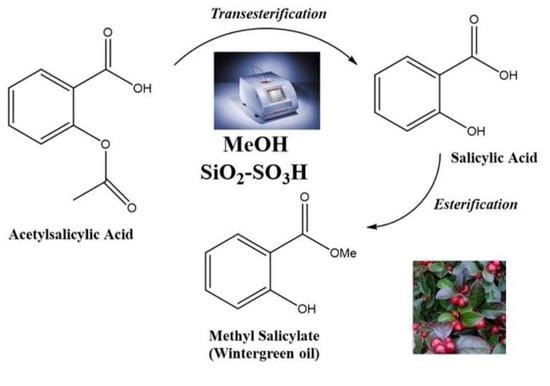
Tandem Transesterification–Esterification Reactions Using a Hydrophilic Sulfonated Silica Catalyst for the Synthesis of Wintergreen Oil from Acetylsalicylic Acid Promoted by Microwave Irradiation
Abstract
:1. Introduction
2. Experimental
2.1. Raw Materials and Chemicals
2.2. Instrumentation
2.3. Preparation of the Silica Gel and Sulfonated Silica (SiO2–SO3H)
2.4. Typical Procedures
2.4.1. Tandem Esterification and Transesterification of ASA in MeOH Using SiO2–SO3H as the Catalyst
2.4.2. Esterification (Direct Methylation) of SA in MeOH Using SiO2–SO3H as the Catalyst
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tyler, V.E.; Brady, L.; Robbers, J.E. Volatile oils. In Pharmacognosy, 8th ed.; Lea & Febiger: Philadelphia, PA, USA, 1981; pp. 103–143. [Google Scholar]
- Ojha, P.K.; Poudel, D.K.; Dangol, S.; Rokaya, A.; Timsina, S.; Satyal, P.; Setzer, W.N. Volatile Constituent Analysis of Wintergreen Essential Oil and Comparison with Synthetic Methyl Salicylate for Authentication. Plants 2022, 11, 1090. [Google Scholar] [CrossRef] [PubMed]
- James, D.G.; Price, T.S. Field-Testing of Methyl Salicylate for Recruitment and Retention of Beneficial Insects in Grapes and Hops. J. Chem. Ecol. 2004, 30, 1613–1628. [Google Scholar] [CrossRef] [PubMed]
- Shulaev, V.; Silverman, P.; Raskin, I. Airborne signalling by methyl salicylate in plant pathogen resistance. Nature 1997, 385, 718–721. [Google Scholar] [CrossRef]
- Koay, J.B.; Natasya, N.N.; Nashithatul, M.A.G.; Ihsanuddin, R.; Salleh, F.M.; Azil, A.H. Using wintergreen oil for mounting mosquito larvae: A safer alternative to xylene. Biotech. Histochem. 2016, 91, 63–70. [Google Scholar] [CrossRef]
- Park, S.-W.; Liu, P.-P.; Forouhar, F.; Vlot, A.C.; Tong, L.; Tietjen, K.; Klessig, D.F. Use of a Synthetic Salicylic Acid Analog to Investigate the Roles of Methyl Salicylate and Its Esterases in Plant Disease Resistance. J. Biol. Chem. 2009, 284, 7307–7317. [Google Scholar] [CrossRef] [Green Version]
- Buckingham, J.; Macdonald, F. Dictionary of Organic Compounds, 6th ed.; Chapman & Hall: London, UK, 1998. [Google Scholar]
- Ribnicky, D.M.; Poulev, A.; Raskin, I. The Determination of Salicylates in Gaultheria procumbens for Use as a Natural Aspirin Alternative. J. Nutraceuticals Funct. Med. Foods 2003, 4, 39–52. [Google Scholar] [CrossRef]
- Murphy, B.J.; Carlson, R.E.; Howa, J.D.; Wilson, T.M.; Buch, R.M. Determining the authenticity of methyl salicylate in Gaultheria procumbens L. and Betula lenta L. essential oils using isotope ratio mass spectrometry. J. Essent. Oil Res. 2021, 33, 442–451. [Google Scholar] [CrossRef]
- Zanger, M.; McKee, J.R. The synthesis of methyl salicylate: Amine diazotization. J. Chem. Educ. 1988, 65, 1106. [Google Scholar] [CrossRef]
- Molleti1, J.; Yadav, G.D. Green synthesis of methyl salicylate using novel sulfated iron oxide–zirconia catalyst. Clean Technol. Environ. Policy 2019, 21, 533–545. [Google Scholar] [CrossRef]
- Sreekumar, K.; Jyothi, T.M.; Mathew, T.; Talawarc, M.B.; Sugunana, S.; Raob, B.S. Selective N-methylation of aniline with dimethyl carbonate over Zn(1−x)Co(x)Fe2O4 (x = 0, 0.2, 0.5, 0.8 and 1.0) type systems. J. Mol. Catal. A Chem. 2000, 159, 327–334. [Google Scholar] [CrossRef]
- Kirumakki, S.R.; Nagaraju, N.; Murthy, K.V.; Narayanan, S. Esterification of salicylic acid over zeolites using dimethyl carbonate. Appl. Catal. A Gen. 2002, 226, 175–182. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, J.; Zhao, N.; Wei, W.; Suna, Y. One-pot synthesis of mesostructured AlSBA-15-SO3H effective catalysts for the esterification of salicylic acid with dimethyl carbonate. Microporous Mesoporous Mater. 2006, 92, 195–200. [Google Scholar] [CrossRef]
- Su, X.; Li, J.; Xiao, F.; Wei, W.; Sun, Y. Esterification of Salicylic Acid with Dimethyl Carbonate over Mesoporous Aluminosilicate. Ind. Eng. Chem. Res. 2009, 48, 3685–3691. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, W.S.; Li, H.M.; Shi, H.; Yan, Y.S.; Wang, Z.G. Esterification of salicylic acid using Ce4+ modified cation-exchange resin as catalyst. J. Chil. Chem. Soc. 2012, 57, 1477–1481. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Zhu, W.; Li, H.; Liu, H.; Zhang, M.; Yan, Y.; Wang, Z. Microwave-accelerated esterification of salicylic acid using Brönsted acidic ionic liquids as catalysts. Catal. Commun. 2010, 11, 588–591. [Google Scholar] [CrossRef]
- Major, B.; Kelemen-Horváth, I.; Csanádi, Z.; Bélafi-Bakó, K.; Gubicza, L. Microwave assisted enzymatic esterification of lactic acid and ethanol in phosphonium type ionic liquids as co-solvents. Green Chem. 2009, 11, 614–616. [Google Scholar] [CrossRef]
- Liao, X.J.; Raghavan, G.S.V.; Yaylayan, V.A. A novel way to prepare n-butylparaben under microwave irradiation. Tetrahedron Lett. 2002, 43, 45–48. [Google Scholar] [CrossRef]
- Barbosa, S.L.; Ottone, M.; Santos, M.C.; Gelson, C., Jr.; Lima, C.D.; Glososki, G.C.; Lopes, N.P.; Klein, S.I. Benzyl benzoate and dibenzyl ether from of benzoic acid and benzyl alcohol under microwave irradiation using a SiO2–SO3H catalyst. Catal. Commun. 2015, 68, 97–100. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, S.L.; Freitas, M.S.; dos Santos, W.T.P.; Nelson, D.L.; Klein, S.I.; Clososki, G.C.; Caires, F.J.; Baroni, A.C.M.; Wentz, A.P. Dehydration of D-fructose to 5-hydroxymethyl-2-furfural in DMSO using a hydrophilic sulfonated silica catalyst in a process promoted by microwave irradiation. Sci. Rep. 2021, 11, 1919. [Google Scholar] [CrossRef]
- Hartel, A.M.; Hanna, J.M., Jr. Preparation of Oil of Wintergreen from Commercial Aspirin Tablets. A Microscale Experiment Highlighting Acyl Substitutions. J. Chem. Educ. 2009, 86, 475–476. [Google Scholar] [CrossRef]
- Yadav, G.D.; Mehta, P.H. Heterogeneous catalysis in esterification reactions: Preparation of phenethyl acetate and cyclohexyl acetate by using a variety of solid acidic catalysts. Ind. Eng. Chem. Res. 1994, 33, 2198–2208. [Google Scholar] [CrossRef]
- Khan, Z.; Javed, F.; Shamair, Z.; Hafeez, A.; Fazal, T.; Aslam, A.; Zimmerman, W.B.; Rehman, F. Current Developments in Esterification Reaction: A Review on Process and Parameters. J. Ind. Eng. Chem. 2021, 103, 80–101. [Google Scholar] [CrossRef]
- Barbosa, S.L.; Rocha, A.C.P.; Nelson, D.L.; Freitas, M.S.; Mestre, A.A.P.F.; Klein, S.I.; Clososki, G.C.; Caires, F.J.; Flumignan, D.L.; dos Santos, L.K.; et al. Catalytic Transformation of Triglycerides to Biodiesel with SiO2-SO3H and Quaternary Ammonium Salts in Toluene or DMSO. Molecules 2022, 27, 953. [Google Scholar] [CrossRef]
- Available online: https://origin-www.thermofisher.com/us/en/home/industrial/spectroscopy-elemental-isotope-analysis/spectroscopy-elemental-isotope-analysis-learning-center/spectroscopy-elemental-isotope-analysis-resource/library/nmr-tech-talk/nmr-tech-talk-march-2015/nmr-spectrum-aspirin.html (accessed on 3 May 2022).
- Jencks, W.P. Catalysis in Chemistry and Enzymology; Dover Publications, Inc.: New York, NY, USA, 1989; Chapter 1. [Google Scholar]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]




| Entry a | MeOH (mL) | SiO2–SO3H Catalyst (%) | Time (min) | Temperature (°C) | Yield (%) |
|---|---|---|---|---|---|
| 1 | 1.0 | 10 | 10 | 120 | 60 |
| 2 | 1.0 | 10 | 20 | 120 | 56 |
| 3 | 1.0 | 20 | 5 | 120 | 23 |
| 4 | 0.5 | 20 | 10 | 120 | 52 |
| 5 | 1.0 | 20 | 10 | 120 | 62 |
| 6 | 1.0 | 20 | 20 | 120 | 54 |
| 7 | 2.0 | 20 | 20 | 120 | 37 |
| 8 b | 1.0 | 20 | 40 | 120 | 94 |
| 8′ b | 1.0 | 20 | 40 | 120 | 93 |
| 8″ b | 1.0 | 20 | 40 | 120 | 93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, S.L.; Nelson, D.L.; Freitas, M.d.S.; dos Santos, W.T.P.; Klein, S.I.; Clososki, G.C.; Caires, F.J.; Wentz, A.P. Tandem Transesterification–Esterification Reactions Using a Hydrophilic Sulfonated Silica Catalyst for the Synthesis of Wintergreen Oil from Acetylsalicylic Acid Promoted by Microwave Irradiation. Molecules 2022, 27, 4767. https://doi.org/10.3390/molecules27154767
Barbosa SL, Nelson DL, Freitas MdS, dos Santos WTP, Klein SI, Clososki GC, Caires FJ, Wentz AP. Tandem Transesterification–Esterification Reactions Using a Hydrophilic Sulfonated Silica Catalyst for the Synthesis of Wintergreen Oil from Acetylsalicylic Acid Promoted by Microwave Irradiation. Molecules. 2022; 27(15):4767. https://doi.org/10.3390/molecules27154767
Chicago/Turabian StyleBarbosa, Sandro L., David Lee Nelson, Milton de S. Freitas, Wallans Torres Pio dos Santos, Stanlei I. Klein, Giuliano C. Clososki, Franco J. Caires, and Alexandre P. Wentz. 2022. "Tandem Transesterification–Esterification Reactions Using a Hydrophilic Sulfonated Silica Catalyst for the Synthesis of Wintergreen Oil from Acetylsalicylic Acid Promoted by Microwave Irradiation" Molecules 27, no. 15: 4767. https://doi.org/10.3390/molecules27154767
APA StyleBarbosa, S. L., Nelson, D. L., Freitas, M. d. S., dos Santos, W. T. P., Klein, S. I., Clososki, G. C., Caires, F. J., & Wentz, A. P. (2022). Tandem Transesterification–Esterification Reactions Using a Hydrophilic Sulfonated Silica Catalyst for the Synthesis of Wintergreen Oil from Acetylsalicylic Acid Promoted by Microwave Irradiation. Molecules, 27(15), 4767. https://doi.org/10.3390/molecules27154767