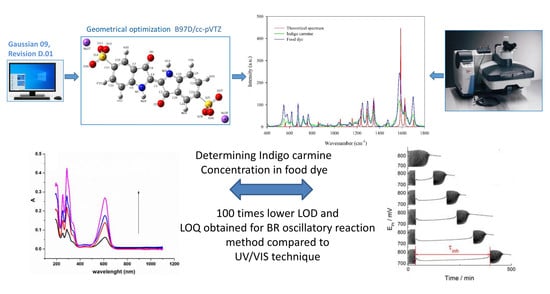
Indigo Carmine in a Food Dye: Spectroscopic Characterization and Determining Its Micro-Concentration through the Clock Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Infra-Red (IR) Spectroscopy
2.1.1. Attenuated Total Reflection (ATR)-Fourier-Transform Infrared (FTIR) Spectroscopy
2.1.2. FTIR Spectroscopy-KBr Pellet
2.2. Raman Spectroscopy
2.2.1. Micro Raman Spectroscopy
2.2.2. Raman-Spectrum Optimization and Calculation
2.3. UV-Vis Measurements
2.3.1. The Standard Calibration Curve of Indigo Carmine
2.3.2. The D-Glucose UV/Vis Spectrum
2.3.3. The Food-Dye UV/Vis Spectrum
2.4. The Oscillatory Briggs-Rauscher Reaction and Experimental Conditions
- Pure D-glucose (100 μL of 0.1 mol dm−3 aqueous solution) was injected;
- the food dye dissolved in bi-distilled water indigo carmine concentrations in BR solution from 2.62 × 10−7 mol dm−3 to 1.04 × 10−6 mol dm−3 was examined.
3. Results and Discussion
3.1. ATR FTIR Spectroscopy and KBr FTIR Technique
3.2. Raman Spectroscopy
3.3. UV/Vis and Briggs-Rauscher Methods
3.4. The Degradation of Indigo Carmine in a Briggs-Rauscher Oscillatory Reaction and Its Remarkable Sensitivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dilrukshi, P.G.T.; Munasinghe, H.; Silva, A.B.G.; De Silva, P.G.S.M. Identification of Synthetic Food Colours in Selected Confectioneries and Beverages in Jaffna District, Sri Lanka. J. Food Qual. 2019, 2019, 7453169. [Google Scholar] [CrossRef]
- Lok, K.Y.-W.; Chung, W.-Y.; Benzie, I.F.F.; Woo, J. Colour additives in snack foods consumed by primary school children in Hong Kong. Food Addit. Contam. 2010, 3, 148–155. [Google Scholar] [CrossRef]
- Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives (OJ L 354, 31.12.2008. pp. 16–33. Available online: https://eur-lex.europa.eu/eli/reg/2008/1333/ (accessed on 20 June 2022).
- Šuleková, M.; Smrčová, M.; Hudák, A.; Heželová, M.; Fedorová, M. Organic colouring agents in the pharmaceutical industry. Folia Vet. 2017, 61, 32–46. [Google Scholar] [CrossRef] [Green Version]
- Rutter, M.D.; Saunders, B.P.; Schofield, G.; Forbes, A.; Price, A.B.; Talbot, I.C. Pancolonic indigo carmine dye spraying for the detection of dysplasia in ulcerative colitis. Gut 2004, 53, 256–260. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food additives and Nutrient Sources added to Food (ANS), Scientific Opinion on the re-evaluation of Indigo Carmine (E 132) as a food additive. EFSA J. 2014, 12, 3768. [CrossRef]
- Jeon, H.J.; Yoon, J.S.; Cho, S.S.; Kang, K.O. Indigo carmine-induced hypotension in patients undergoing general anaesthesia. Singap. Med. J. 2012, 53, e57–e59. [Google Scholar]
- Sari, M.M. Removal of acidic indigo carmine textile dye from aqueous solutions using radiation induced cationic hydrogels. Water Sci. Technol. 2010, 61, 2097–2104. [Google Scholar] [CrossRef]
- Babu, A.N.; Reddy, D.S.; Sharma, P.; Kumar, G.S.; Ravindhranath, K.; Krishna Mohan, G.V. Removal of Hazardous Indigo Carmine Dye from Waste Water Using Treated Red Mud. Mater. Today Proc. 2019, 17, 198–208. [Google Scholar] [CrossRef]
- Bentouami, A.; Ouali, M.S.; De Menorval, L.-C. Photocatalytic decolourization of indigo carmine on 1, 10-phenanthrolinium intercalated bentonite under UV-B and solar irradiation. J. Photochem. Photobiol. A Chem. 2010, 212, 101–106. [Google Scholar] [CrossRef]
- Vautier, M.; Guillard, C.; Herrmann, J.-M. Photocatalytic Degradation of Dyes in Water: Case Study of Indigo and of Indigo Carmine. J. Catal. 2001, 201, 46–59. [Google Scholar] [CrossRef]
- Raji, Y.; Mechnou, I.; Yassine, W.; Kadri, Z.; Oumghar, K.; Cherkaoui, O.; Zyade, S. Extraction of the natural indigo carmine pigment from the Isatis plant, characterization and dyeing of wool. IOP Conf. Ser. Mater. Sci. Eng. 2020, 948, 012017. [Google Scholar] [CrossRef]
- Altunay, N. An optimization approach for fast, simple and accurate determination of indigo-carmine in food samples, Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 257, 119791. [Google Scholar] [CrossRef]
- Berzas Nevado, J.J.; Rodríguez Flores, J.; Villaseñor Llerena, M.J.; Rodríguez Fariñas, N. Simultaneous spectrophotometric determination of tartrazine, patent blue V, and indigo carmine in commercial products by partial least squares and principal component regression methods. Talanta 1999, 48, 895–903. [Google Scholar] [CrossRef]
- Debina, B.; Eric, S.N.; Fotio, D.; Arnaud, K.T.; Lemankreo, D.-Y.; Rahman, A.N. Adsorption of Indigo Carmine Dye by Composite Activated Carbons Prepared from Plastic Waste (PET) and Banana Pseudo Stem. J. Mater. Sci. Eng. 2020, 8, 39–55. [Google Scholar] [CrossRef]
- Pourreza, N.; Ghomi, M. Simultaneous cloud point extraction and spectrophotometric determination of carmoisine and brilliant blue FCF in food samples. Talanta 2011, 84, 240–243. [Google Scholar] [CrossRef]
- Tikhomirova, T.I.; Ramazanova, G.R.; Apyari, V.V. Adsorption Preconcentration of Synthetic Anionic Food Dyes. J. Anal. Chem. 2017, 72, 779–800. [Google Scholar] [CrossRef]
- Arvand, M.; Saberi, M.; Ardaki, M.S.; Mohammadi, A. Mediated electrochemical method for the determination of indigo carmine levels in food products. Talanta 2017, 173, 60–68. [Google Scholar] [CrossRef]
- Deroco, P.B.; Medeiros, R.A.; Rocha-Filho, R.C.; Fatibello-Filho, O. Selective and simultaneous determination of indigo carmine and allura red in candy samples at the nano-concentration range by flow injection analysis with multiple pulse amperometric detection. Food Chem. 2017, 247, 66–72. [Google Scholar] [CrossRef]
- López-de-Alba, P.L.; López-Martínez, L.; De-León-Rodríguez, L.M. Simultaneous Determination of Synthetic Dyes Tartrazine, Allura Red and Sunset Yellow by Differential Pulse Polarography and Partial Least Squares. A Multivariate Calibration Method. Electroanalysis 2002, 14, 197–205. [Google Scholar] [CrossRef]
- Alves, S.P.; Brum, D.M.; Branco de Andrade, É.C.; Pereira Netto, A.D. Determination of synthetic dyes in selected foodstuffs by high performance liquid chromatography with UV-DAD detection. Food Chem. 2008, 107, 489–496. [Google Scholar] [CrossRef]
- Floriano, L.; Ribeiro, L.C.; Saibt, N.; Bandeira, N.M.G.; Prestes, O.D.; Zanella, R. Determination of six synthetic dyes in sports drinks by dispersive solid-phase extraction and HPLC-UV-Vis. J. Braz. Chem. Soc. 2018, 29, 602–608. [Google Scholar] [CrossRef]
- García-Falcón, M.S.; Simal-Gándara, J. Determination of food dyes in soft drinks containing natural pigments by liquid chromatography with minimal clean-up. Food Control 2005, 16, 293–297. [Google Scholar] [CrossRef]
- Michalec, K.; Kusior, A. From Adsorbent to Photocatalyst: The Sensitization Effect of SnO2 Surface towards Dye Photodecomposition. Molecules 2021, 26, 7123. [Google Scholar] [CrossRef] [PubMed]
- de Sá, I.C.; Feiteira, F.N.; Pacheco, W.F. Quantification of the Food Dye Indigo Carmine in Candies Using Digital Image Analysis in a Polyurethane Foam Support. Food Anal. Methods 2020, 13, 962–969. [Google Scholar] [CrossRef]
- Roggo, Y.; Edmond, A.; Chalus, P.; Ulmschneider, M. Infrared hyperspectral imaging for qualitative analysis of pharmaceutical solid forms. Anal. Chim. Acta 2005, 535, 79–87. [Google Scholar] [CrossRef]
- Molla, A.; Youk, J.H. Chemical clock reactions with organic dyes: Perspective, progress, and applications. Dyes Pigment. 2022, 202, 110237. [Google Scholar] [CrossRef]
- Briggs, T.S.; Rauscher, W.C. An oscillating iodine clock. J. Chem. Educ. 1973, 50, 496. [Google Scholar] [CrossRef]
- Zhou, Y.; Uddin, W.; Hu, G. Kinetic identification of three metal ions by using a Briggs-Rauscher oscillating system. Microchem. J. 2021, 160, 105617. [Google Scholar] [CrossRef]
- Zhang, L.; Uddin, W.; Hu, G.; Shen, X.; Hu, L. A method to distinguish halide ions by using a Briggs-Rauscher reaction. Microchem. J. 2021, 168, 106380. [Google Scholar] [CrossRef]
- Cervellati, R.; Höner, K.; Furrow, S.D.; Neddens, C.; Costa, S. The Briggs-Rauscher Reaction as a Test to Measure the Activity of Antioxidants. Helv. Chem. Acta 2001, 84, 3533–3547. [Google Scholar] [CrossRef]
- Cervellati, R.; Renzulli, C.; Guerra, M.C.; Speroni, E. Evaluation of antioxidant activity of some natural polyphenolic compounds using the Briggs–Rauscher reaction method. J. Agric. Food Chem. 2002, 50, 7504–7509. [Google Scholar] [CrossRef]
- Cervellati, R.; Höner, K.; Furrow, S.D.; Mazzanti, F. Inhibitory effects by antioxidants on the oscillations of the Briggs-Rauscher reaction in mixed EtOH/H2O medium. Helv. Chem. Acta 2002, 85, 2523–2537. [Google Scholar] [CrossRef]
- Furrow, S.D.; Höner, K.; Cervellati, R. Inhibitory Effects by Ascorbic Acid on the Oscillations of the Briggs-Rauscher Reaction. Helv. Chem. Acta 2004, 87, 735–741. [Google Scholar] [CrossRef]
- Cervellati, R.; Furrow, S.D. Perturbations of the Briggs–Rauscher oscillating system by iron–phenanthroline complexes. Inorg. Chim. Acta 2007, 360, 842–848. [Google Scholar] [CrossRef]
- Furrow, S.; Greco, E.; Venturi, M.L.; Cervellati, R. Perturbations of an Acetone-Based Briggs–Rauscher System by α-Tocopherol (Vitamin, E.). Helv. Chem. Acta 2010, 93, 837–846. [Google Scholar] [CrossRef]
- Maksimović, T.V.; Maksimović, J.P.; Joksović, L.G.; Nedić, Z.P.; Pagnacco, M.C. Oscilatorna reakcija kao sistem detektor za dopirane i nedopirane fosfat-volframove bronze. Hem. Ind. 2018, 72, 275–283. [Google Scholar] [CrossRef] [Green Version]
- Pagnacco, M.C.; Maksimović, J.P.; Mudrinić, T.M.; Mojović, Z.D.; Nedić, Z.P. Briggs-Rauscher reaction as a novel electrochemical detector for phosphate tungsten and phosphate molybdenum bronzes. J. Electroanal. Chem. 2019, 849, 113369. [Google Scholar] [CrossRef]
- Maksimović, T.V.; Maksimović, J.P.; Tančić, P.I.; Potkonjak, N.I.; Nedić, Z.P.; Joksović, L.G.; Pagnacco, M.C. A Possible Connection between Phosphate Tungsten Bronzes Properties and Briggs-Rauscher Oscillatory Reaction Response. Sci. Sinter. 2021, 53, 223–235. [Google Scholar] [CrossRef]
- Pagnacco, M.C.; Maksimović, J.P.; Mudrinić, T.M.; Banković, P.T.; Nedić-Vasiljević, B.M.; Milutinović-Nikolić, A.D. Oscillatory Briggs-Rauscher Reaction as “Fingerprint” for Bentonite Identification: The Fine-Tuning of Oscillatory Dynamics with Addition of Clay. ChemistrySelect 2020, 5, 8137–8141. [Google Scholar] [CrossRef]
- Peica, N.; Kiefer, W. Characterization of indigo carmine with surface-enhanced resonance Raman spectroscopy (SERRS) using silver colloids and island films, and theoretical calculations. J. Raman Spectrosc. 2008, 39, 47–60. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, D.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Doménech-Carbó, M.T.; Álvarez-Romero, C.; Doménech-Carbó, A.; Osete-Cortina, L.; Martínez-Bazán, M.L. Microchemical surface analysis of historic copper-based coins by the combined use of FIB-FESEM-EDX, OM, FTIR spectroscopy and solid-state electrochemical techniques. Microchem. J. 2019, 148, 573–581. [Google Scholar] [CrossRef]
- Sencanski, J.; Nikolić, N.; Nedić, Z.; Maksimović, J.; Blagojević, S.; Pagnacco, M. Natural Pigment from Madder Plant as an Eco-Friendly Cathode Material for Aqueous Li and Na-Ion Batteries. J. Electrochem. Soc. 2021, 168, 100535. [Google Scholar] [CrossRef]
- Ju, Z.; Sun, J.; Liu, Y. Molecular Structures and Spectral Properties of Natural Indigo and Indirubin: Experimental and DFT Studies. Molecules 2019, 24, 3831. [Google Scholar] [CrossRef] [Green Version]
- Del Rıo, M.S.; Picquart, M.; Haro-Poniatowski, E.; van Elslande, E.; Uc, V.H. On the Raman spectrum of Maya blue. J. Raman Spectrosc. 2006, 37, 1046–1053. [Google Scholar] [CrossRef]
- Baran, A.; Fiedler, A.; Schulzband, H.; Baranska, M. In situ Raman and IR spectroscopic analysis of indigo dye. Anal. Methods 2010, 2, 1372–1376. [Google Scholar] [CrossRef]
- Volkov, V.V.; Chelli, R.; Righini, R.; Perry, C.C. Indigo chromophores and pigments: Structure and dynamics. Dyes Pigment. 2020, 172, 107761. [Google Scholar] [CrossRef]
- Karapanayiotis, T.; Jorge Villar, S.E.; Bowen, R.D.; Edwards, H.G.M. Raman spectroscopic and structural studies of indigo and its four 6,6A-dihalogeno analogues. Analyst 2004, 129, 613–618. [Google Scholar] [CrossRef]
- Tatsch, E.; Schrader, B. Near-Infrared Fourier Transform Raman Spectroscopy of Indigoids. J. Raman Spectrosc. 1995, 26, 467–473. [Google Scholar] [CrossRef]
- Fiedler, A.; Baranska, M.; Schulz, H. FT-Raman spectroscopy—A rapid and reliable quantification protocol for the determination of natural indigo dye in Polygonum tinctorium. J. Raman Spectrosc. 2011, 42, 551–557. [Google Scholar] [CrossRef]
- Shadi, I.T.; Chowdhry, B.Z.; Snowdena, M.J.; Withnall, R. Analysis of the conversion of indigo into indigo carmine dye using SERRS. Chem. Commun. 2004, 12, 1436–1437. [Google Scholar] [CrossRef] [PubMed]
- Abe, J.; Nagasawa, Y.; Takahashi, H. Molecular orbital and resonance Raman studies of the structures of N,N’disubstituted indigo dyes. J. Chem. Phys. 1989, 91, 3431–3434. [Google Scholar] [CrossRef]
- JUICE PRODUCTS, Determination of Synthetic Dyes by High Performance Liquid Chromatography, Moscow Standartinform 2017. pp. 1–13. Available online: https://www.rts-tender.ru/poisk/gost/34229-2017 (accessed on 20 June 2022).
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Stanisavljev, D.R.; Milenković, M.C.; Mojović, M.D.; Popović-Bijelić, A.D. Oxygen Centered Radicals in Iodine Chemical Oscillators. J. Phys. Chem. A 2011, 115, 7955–7958. [Google Scholar] [CrossRef]
- Milenković, M.C.; Stanisavljev, D.R. Role of Free Radicals in Modeling the Iodide–Peroxide Reaction Mechanism. J. Phys. Chem. A 2012, 116, 5541–5548. [Google Scholar] [CrossRef]
- Stanisavljev, D.R.; Milenković, M.C.; Popović-Bijelić, A.D.; Mojović, M.D. Radicals in the Bray–Liebhafsky Oscillatory Reaction. J. Phys. Chem. A 2013, 117, 3292–3295. [Google Scholar] [CrossRef]
- Milenkovic, M.; Potkonjak, N. The effect of hydroxycinnamic acids on oxy-radical generating iodide-hydrgen peroxide reaction. Bull. Chem. Soc. Jpn. 2014, 87, 1255–1259. [Google Scholar] [CrossRef]
- Pagnacco, M.C.; Mojović, M.D.; Popović Bijelić, A.D.; Horváth, A.K. Investigation of the Halogenate-Hydrogen Peroxide Reactions Using the Electron Paramagnetic Resonance Spin Trapping Technique. J. Phys. Chem. A 2017, 121, 3207–3212. [Google Scholar] [CrossRef]
- Maksimović, J.P.; Tošović, J.; Pagnacco, M.C. Insight into the origin of pyrocatechol inhibition on oscillating Bray-Liebhafsky reaction: Combined experimental and theoretical study. Bull. Chem. Soc. Jpn. 2020, 93, 676–684. [Google Scholar] [CrossRef]
- Muthakia, G.K.; Jonnalagadda, S.B. Kinetics and mechanism of indigo carmine-acidic iodate reaction. An indicator reaction for catalytic determination of Ru(III) ion. Int. J. Chem. Kinet. 1989, 21, 519–533. [Google Scholar] [CrossRef]
- Weisz, H.; Pantel, S.; Marquardt, G. Catalytic-kinetic absorptiostat technique with the indigo carmine-hydrogen peroxide reaction as the indicator reaction. Anal. Chim. Acta 1982, 143, 177–184. [Google Scholar] [CrossRef]
- Dalmázio, I.; De Urzedo, A.P.F.M.; Alves, T.M.A.; Catharino, R.R.; Eberlin, M.N.; Nascentes, C.C.; August, R. Electrospray ionization mass spectroscopy monitoring of indigo carmine degradation by advanced oxidative processes. J. Mass Spectrom. 2007, 42, 1273–1278. [Google Scholar] [CrossRef]
- Chuesaard, T.; Wonganan, T.; Wongchanapiboon, T.; Liawruangrath, S. Reversed flow injection spectrophotometric determination of chlorate. Talanta 2009, 79, 1181–1187. [Google Scholar] [CrossRef]
- Yim, M.B.; Berlett, B.S.; Chock, P.B.; Stadtman, E.R. Manganese(II)-bicarbonate-mediated catalytic activity for hydrogen peroxide dismutation and amino acid oxidation: Detection of free radical intermediates. Proc. Natl. Acad. Sci. USA 1990, 87, 394–398. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, H.A. A Determination of some rate constants for the radical processes in the radiation chemistry of water. J. Phys. Chem. 1962, 66, 255–262. [Google Scholar] [CrossRef]
- Kettle, A.J.; Clark, B.M.; Winterbourn, C.C. Superoxide Converts Indigo Carmine to Isatin Sulfonic Acid: Implications for the Hypothesis that Neutrophils Produce Ozone. J. Biol. Chem. 2004, 279, 18521–18525. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.; Alaam, M.; El-Haes, H.; Jalbout, A.F.; de Leon, A. Analysis of the structure and vibrational spectra of glucose and fructose. Eclet. Quim. 2006, 31, 15–21. [Google Scholar] [CrossRef]
- Korolevich, M.V.; Zhbankov, R.G.; Sivchik, V.V. Calculation of absorption band frequencies and intensities in the IR spectrum of α-d-glucose in a cluster. J. Mol. Struct. 1990, 220, 301–313. [Google Scholar] [CrossRef]





| Food Dye | INDIGO Carmine Exp. | Spectrum Calculated | Band Vibrations from Facio Obtained in This Work | Ref. |
|---|---|---|---|---|
| Spectrum | Spectrum | |||
| Raman shift [1/cm] | Raman shift [1/cm] | |||
| 549 | 552 | 561 | C-C ring bending: weak; C-H bending: weak; N-H bending: weak; S-O bending: weak | [42,48,49] |
| 676 | 674 | 669 | C-C ring bending: weak; C-H bending: weak; N-H bending: weak | [31,48,49] |
| 723 | 723 | 705 | N-H bending: medium; C-H bending: medium; S-C stretching: weak | [49] |
| 766 | 766 | 761 | C-C ring stretching: weak; C-H bending: weak | [42,48,49,50,51,52,53] |
| 869 | 864 | 856 | C-C-C ring bending: weak; C-N bending: weak | [42,49] |
| 1040 | 1030 | 1052 | C-H bending: strong; C-S stretching: medium; C-C ring stretching: weak | [42,48,49] |
| 1137 | 1137 | 1157 | C-H bending: strong; S-O stretching: weak | [48,49,53] |
| 1183 | 1183 | 1157 | C-C ring stretching: medium; C-H bending: strong; N-H bending: weak | [42,48,49,50,51,52] |
| 1248 | 1240 | 1235 | C-H bending: strong; N-C asymmetrical stretching: medium; C-C ring stretching: weak | [42,48,49,50,51,52,53] |
| 1294 | 1296 | 1293 | C-H bending; C-C ring stretching; N-H stretching | [42,48,49,51,53] |
| 1348 | 1346 | 1349 | C3 = C4 stretching: strong; C-C ring stretching; C-N ring stretching; C-H bending | [42,48,51,52] |
| 1442 | 1473 | 1458 | C-C ring stretching; C-H bending: strong C13-H32, strong C24-H36; N-H bending: weak N5-H29, weak N18-H33 | [42,48,49,50,52,53,54] |
| 1578 | 1576 | 1588 | C = C ring-ring stretching: strong C3-C4; C-C ring stretching; C = O stretching; N-H bending | [42,48,49,50,51,52,53,54,55,56] |
| 1625 | 1626 | 1625 | C = C ring-ring stretching: strong C3-C4; C-C ring stretching; C = O ring symmetrical stretching | [42,48,49,50,51,52,53] |
| 1699 | 1697 | 1703 | C = C ring-ring stretching: strong C3-C4; C = O ring symmetrical stretching: strong C2-O1, strong C8-O9 | [42,48,49,50,51,52,53,54,55] |
| UV/Vis Spectrophotometric Method | ||||
|---|---|---|---|---|
| A = n + kC | n | Error n (Sn) | k(slope) (Mol−1 dm3) | Error k (Sk) (Mol−1 dm3) |
| A = 1.000 + 9000 × C | 1.000 | 0.005 | 9000 | 100 |
| LOD = 3.3 × Sn/k | LOQ = 10 × Sn/k | |||
| 1.8 × 10−6 mol dm−3 | 5.7 × 10−6 mol dm−3 | |||
| Briggs–Rauscher oscillatory method | ||||
| τinh = n + kC | n(s) | error n (Sn) (s) | k(slope) (mol−1 dm3 s) | error k (Sk) (mol−1 dm3 s) |
| τinh = 3 + 4 × 108 × Cind | 3 | 2 | 4 × 108 | 1 × 107 |
| LOD = 3.3 × Sn/k | LOQ = 10 × Sn/k | |||
| 1.65 × 10−8 mol dm−3 | 5 × 10−8 mol dm−3 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagnacco, M.C.; Maksimović, J.P.; Nikolić, N.T.; Bajuk Bogdanović, D.V.; Kragović, M.M.; Stojmenović, M.D.; Blagojević, S.N.; Senćanski, J.V. Indigo Carmine in a Food Dye: Spectroscopic Characterization and Determining Its Micro-Concentration through the Clock Reaction. Molecules 2022, 27, 4853. https://doi.org/10.3390/molecules27154853
Pagnacco MC, Maksimović JP, Nikolić NT, Bajuk Bogdanović DV, Kragović MM, Stojmenović MD, Blagojević SN, Senćanski JV. Indigo Carmine in a Food Dye: Spectroscopic Characterization and Determining Its Micro-Concentration through the Clock Reaction. Molecules. 2022; 27(15):4853. https://doi.org/10.3390/molecules27154853
Chicago/Turabian StylePagnacco, Maja C., Jelena P. Maksimović, Nenad T. Nikolić, Danica V. Bajuk Bogdanović, Milan M. Kragović, Marija D. Stojmenović, Stevan N. Blagojević, and Jelena V. Senćanski. 2022. "Indigo Carmine in a Food Dye: Spectroscopic Characterization and Determining Its Micro-Concentration through the Clock Reaction" Molecules 27, no. 15: 4853. https://doi.org/10.3390/molecules27154853
APA StylePagnacco, M. C., Maksimović, J. P., Nikolić, N. T., Bajuk Bogdanović, D. V., Kragović, M. M., Stojmenović, M. D., Blagojević, S. N., & Senćanski, J. V. (2022). Indigo Carmine in a Food Dye: Spectroscopic Characterization and Determining Its Micro-Concentration through the Clock Reaction. Molecules, 27(15), 4853. https://doi.org/10.3390/molecules27154853