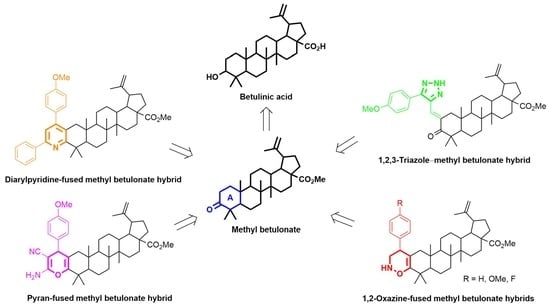
Decoration of A-Ring of a Lupane-Type Triterpenoid with Different Oxygen and Nitrogen Heterocycles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Diastereoselective Michael Addition of Nitromethane to Methyl (E)-2-Benzylidenebetulonate Derivatives towards the Synthesis of 1,2-Oxazine-Fused BoOMe Compounds
2.2. Synthesis of Other Oxygen and Nitrogen Heterocycles-Containing BoOMe Compounds Using Different Synthetic Methodologies
2.2.1. Michael Addition of Malononitrile to Methyl (E)-2-(4-Methoxybenzylidene)betulonate towards the Synthesis of a Pyran-Fused BoOMe Compound
2.2.2. Kröhnke Pyridine Synthesis—Synthesis of a Diarylpyridine-Fused BoOMe Compound
2.2.3. 1,3-Dipolar Cycloaddition of Sodium Azide to a Methyl (E)-2-(3-Phenylprop-2-yn-1-ylidene)betulonate Derivative—Synthesis of a 1,2,3-Triazole–BoOMe Compound
3. Materials and Methods
3.1. General Remarks
3.2. Synthesis of Methyl (E)-2-Benzylidenebetulonate Derivatives 3a-c and Methyl (E)-2-[3-(4-Methoxyphenyl)prop-2-yn-1-ylidene]betulonate (10)
3.3. Synthesis of Methyl 2-(2-Nitro-1-phenylethyl)betulonate Derivatives 4a-c
3.4. Synthesis of 1,2-Oxazine-Fused BoOMe Compounds 5a-c
3.5. Synthesis of the Pyran-Fused BoOMe Compound 6
3.6. Synthesis of the Diarylpyridine-Fused BoOMe Compound 8
3.7. Synthesis of the 1,2,3-Triazole–BoOMe Compound 11
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Moghaddam, M.G.; Ahmad, F.B.H.; Samzadeh-Kermani, A. Biological Activity of Betulinic Acid: A Review. Pharmacol. Pharm. 2012, 3, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Ríos, J.L.; Máñez, S. New Pharmacological Opportunities for Betulinic Acid. Planta Med. 2018, 84, 8–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regueiro-Ren, A.; Liu, Z.; Chen, Y.; Sin, N.; Sit, S.-Y.; Swidorski, J.J.; Chen, J.; Venables, B.L.; Zhu, J.; Nowicka-Sans, B.; et al. Discovery of BMS-955176, a Second Generation HIV-1 Maturation Inhibitor with Broad Spectrum Antiviral Activity. ACS Med. Chem. Lett. 2016, 7, 568–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, D.E.; Salzwedel, K.; Allaway, G.P. Bevirimat: A Novel Maturation Inhibitor for the Treatment of HIV-1 Infection. Antivir. Chem. Chemother. 2008, 19, 107–113. [Google Scholar] [CrossRef]
- Dicker, I.; Jeffrey, J.L.; Protack, T.; Lin, Z.; Cockett, M.; Chen, Y.; Sit, S.-Y.; Gartland, M.; Meanwell, N.A.; Regueiro-Ren, A.; et al. GSK3640254 Is a Novel HIV-1 Maturation Inhibitor with an Optimized Virology Profile. Antimicrob. Agents Chemother. 2022, 66, e01876-21. [Google Scholar] [CrossRef]
- Sousa, J.L.C.; Freire, C.S.R.; Silvestre, A.J.D.; Silva, A.M.S. Recent Developments in the Functionalization of Betulinic Acid and Its Natural Analogues: A Route to New Bioactive Compounds. Molecules 2019, 24, 355. [Google Scholar] [CrossRef]
- Kvasnica, M.; Urban, M.; Dickinson, N.J.; Sarek, J. Pentacyclic triterpenoids with nitrogen- and sulfur-containing heterocycles: Synthesis and medicinal significance. Nat. Prod. Rep. 2015, 32, 1303–1330. [Google Scholar] [CrossRef]
- Borkova, L.; Hodon, J.; Urban, M. Synthesis of Betulinic Acid Derivatives with Modified A-Rings and their Application as Potential Drug Candidates. Asian J. Org. Chem. 2018, 7, 1542–1560. [Google Scholar] [CrossRef]
- Pokorny, J.; Borkova, L.; Urban, M. Click Reactions in Chemistry of Triterpenes—Advances Towards Development of Potential Therapeutics. Curr. Med. Chem. 2018, 25, 636–658. [Google Scholar] [CrossRef]
- Gaonkar, S.L.; Nagaraj, V.U.; Nayak, S. A Review on Current Synthetic Strategies of Oxazines. Mini-Rev. Org. Chem. 2019, 16, 43–58. [Google Scholar] [CrossRef]
- Lathwal, A.; Mathew, B.P.; Nath, M. Syntheses, Biological and Material Significance of Dihydro 1,3 oxazine Derivatives: An Overview. Curr. Org. Chem. 2021, 25, 133–174. [Google Scholar] [CrossRef]
- Gamenara, D.; Heinzen, H.; Moyna, P. Design, synthesis and biological evaluation of new oxazines with potential antiparasitic activity. Tetrahedron Lett. 2007, 48, 2505–2507. [Google Scholar] [CrossRef]
- de Brito, M.R.M.; Pelaez, W.J.; Faillace, M.S.; Militao, G.C.G.; Almeida, J.; Arguello, G.A.; Szakonyi, Z.; Fulop, F.; Salvadori, M.C.; Teixeira, F.S.; et al. Cyclohexene-fused 1,3-oxazines with selective antibacterial and antiparasitic action and low cytotoxic effects. Toxicol. In Vitro 2017, 44, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Thapaliya, E.R.; Mazza, M.M.A.; Cusido, J.; Baker, J.D.; Raymo, F.M. A Synthetic Strategy for the Structural Modification of Photoactivatable BODIPY-Oxazine Dyads. ChemPhotoChem 2020, 4, 332–337. [Google Scholar] [CrossRef]
- Deniz, E.; Tomasulo, M.; Cusido, J.; Sortino, S.; Raymo, F.M. Fast and Stable Photochromic Oxazines for Fluorescence Switching. Langmuir 2011, 27, 11773–11783. [Google Scholar] [CrossRef]
- Kumar, D.; Sharma, P.; Singh, H.; Nepali, K.; Gupta, G.K.; Jain, S.K.; Ntie-Kang, F. The value of pyrans as anticancer scaffolds in medicinal chemistry. RSC Adv. 2017, 7, 36977–36999. [Google Scholar] [CrossRef] [Green Version]
- Auria-Luna, F.; Fernández-Moreira, V.; Marqués-López, E.; Gimeno, M.C.; Herrera, R.P. Ultrasound-assisted multicomponent synthesis of 4H-pyrans in water and DNA binding studies. Sci. Rep. 2020, 10, 11594. [Google Scholar] [CrossRef] [PubMed]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Subbaiah, M.A.M.; Meanwell, N.A. Bioisosteres of the Phenyl Ring: Recent Strategic Applications in Lead Optimization and Drug Design. J. Med. Chem. 2021, 64, 14046–14128. [Google Scholar] [CrossRef]
- Haavikko, R.; Nasereddin, A.; Sacerdoti-Sierra, N.; Kopelyanskiy, D.; Alakurtti, S.; Tikka, M.; Jaffe, C.L.; Yli-Kauhaluoma, J. Heterocycle-fused lupane triterpenoids inhibit Leishmania donovani amastigotes. MedChemComm 2014, 5, 445–451. [Google Scholar] [CrossRef] [Green Version]
- Kumari, S.; Carmona, A.V.; Tiwari, A.K.; Trippier, P.C. Amide Bond Bioisosteres: Strategies, Synthesis, and Successes. J. Med. Chem. 2020, 63, 12290–12358. [Google Scholar] [CrossRef]
- Sousa, J.L.C.; Gonçalves, C.; Ferreira, R.M.; Cardoso, S.M.; Freire, C.S.R.; Silvestre, A.J.D.; Silva, A.M.S. Functionalization of Betulinic Acid with Polyphenolic Fragments for the Development of New Amphiphilic Antioxidants. Antioxidants 2021, 10, 148. [Google Scholar] [CrossRef]
- Resende, D.I.S.P.; Guieu, S.; Oliva, C.G.; Silva, A.M.S. Efficient Synthesis of Highly Enantioenriched Δ1-Pyrrolines. Synlett 2015, 26, 846–850. [Google Scholar]
- Oliva, C.G.; Silva, A.M.S.; Resende, D.I.S.P.; Paz, F.A.A.; Cavaleiro, J.A.S. Highly Enantioselective 1,4-Michael Additions of Nucleophiles to Unsaturated Aryl Ketones with Organocatalysis by Bifunctional Cinchona Alkaloids. Eur. J. Org. Chem. 2010, 2010, 3449–3458. [Google Scholar] [CrossRef]
- Hu, Z.-P.; Lou, C.-L.; Wang, J.-J.; Chen, C.-X.; Yan, M. Organocatalytic Conjugate Addition of Malononitrile to Conformationally Restricted Dienones. J. Org. Chem. 2011, 76, 3797–3804. [Google Scholar] [CrossRef]
- Albuquerque, H.M.T.; Santos, C.M.M.; Balanay, M.P.; Cavaleiro, J.A.S.; Silva, A.M.S. 1,6-Conjugate Additions of Carbon Nucleophiles to 2-[(1E,3E)-4-Arylbuta-1,3-dien-1-yl]-4H-chromen-4-ones. Eur. J. Org. Chem. 2017, 5293–5305. [Google Scholar] [CrossRef] [Green Version]
- Tashrifi, Z.; Mohammadi-Khanaposhtani, M.; Hamedifar, H.; Larijani, B.; Ansari, S.; Mahdavi, M. Synthesis and pharmacological properties of polysubstituted 2-amino-4H-pyran-3-carbonitrile derivatives. Mol. Divers. 2020, 24, 1385–1431. [Google Scholar] [CrossRef] [PubMed]
- Fontes, L.F.B.; Nunes da Silva, R.; Silva, A.M.S.; Guieu, S. Unsymmetrical 2,4,6-Triarylpyridines as Versatile Scaffolds for Deep-Blue and Dual-Emission Fluorophores. ChemPhotoChem 2020, 4, 5312–5317. [Google Scholar] [CrossRef]
- Albuquerque, H.M.T.; Santos, C.M.M.; Cavaleiro, J.A.S.; Silva, A.M.S. (E)-2-(4-Arylbut-1-en-3-yn-1-yl)chromones as Synthons for the Synthesis of Xanthone-1,2,3-triazole Dyads. Eur. J. Org. Chem. 2015, 2015, 4732–4743. [Google Scholar] [CrossRef] [Green Version]


 ) for Michael adducts 4a-c. Ar = aryl group.
) for Michael adducts 4a-c. Ar = aryl group.
 ) for Michael adducts 4a-c. Ar = aryl group.
) for Michael adducts 4a-c. Ar = aryl group.
 ) observed for 1,2-oxazine-fused BoOMe compounds 5a-c.
) observed for 1,2-oxazine-fused BoOMe compounds 5a-c.
 ) observed for 1,2-oxazine-fused BoOMe compounds 5a-c.
) observed for 1,2-oxazine-fused BoOMe compounds 5a-c.








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, J.L.C.; Albuquerque, H.M.T.; Silvestre, A.J.D.; Silva, A.M.S. Decoration of A-Ring of a Lupane-Type Triterpenoid with Different Oxygen and Nitrogen Heterocycles. Molecules 2022, 27, 4904. https://doi.org/10.3390/molecules27154904
Sousa JLC, Albuquerque HMT, Silvestre AJD, Silva AMS. Decoration of A-Ring of a Lupane-Type Triterpenoid with Different Oxygen and Nitrogen Heterocycles. Molecules. 2022; 27(15):4904. https://doi.org/10.3390/molecules27154904
Chicago/Turabian StyleSousa, Joana L. C., Hélio M. T. Albuquerque, Armando J. D. Silvestre, and Artur M. S. Silva. 2022. "Decoration of A-Ring of a Lupane-Type Triterpenoid with Different Oxygen and Nitrogen Heterocycles" Molecules 27, no. 15: 4904. https://doi.org/10.3390/molecules27154904
APA StyleSousa, J. L. C., Albuquerque, H. M. T., Silvestre, A. J. D., & Silva, A. M. S. (2022). Decoration of A-Ring of a Lupane-Type Triterpenoid with Different Oxygen and Nitrogen Heterocycles. Molecules, 27(15), 4904. https://doi.org/10.3390/molecules27154904