Chemical Modulators for Targeting Autism Spectrum Disorders: From Bench to Clinic
Abstract
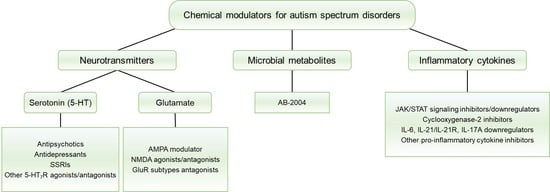
:1. Introduction
2. Pathophysiology of ASD
3. Classification of Chemical Modulators
3.1. Neurotransmitters
3.1.1. 5-HT Receptors (5-HTRs)
3.1.2. Glutamate Receptors (GluRs)
| Compounds | Structures | Targets | Stage | Effects |
|---|---|---|---|---|
| Cx546 |  | AMPA receptor positive allosteric modulator | Preclinical | Improved social interaction [75] |
| Memantine/Memantine hydrochloride (Namenda) |  | NMDA antagonist | Clinical | Improvements in language function, social behavior, ADHD, anxiety and self-stimulatory behaviors [76,77,78] |
| D-cycloserine |  | Partial agonist at NMDA receptors | Preclinical | Positive effects on social behavior [79,80] |
| Clinical | Positive effects on social behavior [81,82], increasing the sustained benefit from short-term social skills intervention [83] | |||
| Fenobam |  | Metabotropic GluR5 antagonist | Preclinical | Reduction of repetitive behaviors, improved social behaviors [86] |
| JNJ16259685 |  | GluR1 antagonist | Preclinical | Reduction of repetitive behaviors, improved social behaviors [86] |
| MPEP |  | Metabotropic GluR5 antagonist | Preclinical | Improved repetitive behavior [87,88] |
| Acamprosate |  | A weak NMDA receptor antagonist, metabotropic GluR5 antagonist | Clinical | Positive effects on verbalization, attention, social behavior and hyperactivity [89] |
3.2. Microbial Metabolites
3.3. Inflammatory Cytokines
| Compounds | Structures | Targets | Stage | Effects |
|---|---|---|---|---|
| Luteolin |  | Inhibitor of neuronal IL-6-induced JAK3/STAT3 phosphorylation | Clinical | Improved eye contact, attention to directions, social interactions [122], improvement in adaptive functioning and overall ASD behavior [123] |
| Diosmin |  | Inhibitor of neuronal IL-6-induced JAK3/STAT3 phosphorylation | Preclinical | Opposed abnormal behavior and neuropathological abnormalities [121] |
| Tyrphostin AG126 |  | Protein tyrosine kinase inhibitor, IL-21/IL-21R, IL-17A, JAK/STAT downregulator | Preclinical | Improvement of repetitive and social behavior [126,127] |
| Resveratrol |  | IL-17A, IL-6, IFN-γ, TNF-α, and JAK1/STAT3 downregulator | Preclinical | Improvement in repetitive behavior, social deficits [129,130,131,132,133] |
| Clinical | Improvement in hyperactivity [134] | |||
| Celecoxib |  | Cyclooxygenase-2 inhibitor | Clinical | Improvement in social deficit, stereotyped activity, and irritability [136] |
| Pentoxifylline |  | Pro-inflammatory cytokine inhibitor | Clinical | Improved social withdrawal/lethargy, stereotyped behavior, irritability, hyperactivity/noncompliance, and inappropriate speech [137] |
| Suramin |  | P2-purinoceptor antagonist, leads to IL-6 decrease | Preclinical | Improvement in sociability [140,141,142] and anxiety [140] |
| Clinical | Improvement in sociability, language, and repetitive/restricted behaviors [143] |
4. Challenges and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nightingale, S. Autism Spectrum Disorders. Nat. Rev. Drug Discov. 2012, 11, 745–746. [Google Scholar] [CrossRef] [PubMed]
- Chadman, K.K.; Guariglia, S.R.; Yoo, J.H. New directions in the treatment of autism spectrum disorders from animal model research. Expert Opin. Drug Discov. 2012, 7, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Avramets, D.; Jeon, B.; Choo, H. Modulation of Serotonin Receptors in Neurodevelopmental Disorders: Focus on 5-HT7 Receptor. Molecules 2021, 26, 3348. [Google Scholar] [CrossRef] [PubMed]
- Baranova, J.; Dragunas, G.; Botellho, M.C.S.; Ayub, A.L.P.; Bueno-Alves, R.; Alencar, R.R.; Papaiz, D.D.; Sogayar, M.C.; Ulrich, H.; Correa, R.G. Autism Spectrum Disorder: Signaling Pathways and Prospective Therapeutic Targets. Cell. Mol. Neurobiol. 2021, 41, 619–649. [Google Scholar] [CrossRef]
- Zeidan, J.; Fombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.S.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global prevalence of autism: A systematic review update. Autism Res. 2022, 15, 778–790. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (Dsm-5®); American Psychiatric Publishing: Washington, DC, USA, 2019; pp. 50–59. [Google Scholar]
- Barton, M.; Volkmar, F. How Commonly Are Known Medical Conditions Associated with Autism? J. Autism Dev. Disord. 1998, 28, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.S.; Needham, B.D.; Meyer, C.R.; Tan, J.; Conrad, M.; Preston, G.M.; Bolognani, F.; Rao, S.G.; Heussler, H.; Griffith, R.; et al. Safety and target engagement of an oral small-molecule sequestrant in adolescents with autism spectrum disorder: An open-label phase 1b/2a trial. Nat. Med. 2022, 28, 528–534. [Google Scholar] [CrossRef]
- Hughes, J.P.; Rees, S.; Kalindjian, S.B.; Philpott, K.L. Principles of Early Drug Discovery. Br. J. Pharmacol. 2011, 162, 1239–1249. [Google Scholar] [CrossRef] [Green Version]
- DeFilippis, M.; Wagner, K.D. Treatment of Autism Spectrum Disorder in Children and Adolescents. Psychopharmacol. Bull. 2016, 46, 18–41. [Google Scholar]
- Posey, D.J.; McDougle, C.J. Pharmacotherapeutic management of autism. Expert Opin. Pharmacother. 2001, 2, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Oswald, D.P.; Sonenklar, N.A. Medication Use Among Children with Autism Spectrum Disorders. J. Child Adolesc. Psychopharmacol. 2007, 17, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Barnard, L.; Young, A.H.; Pearson, J.; Geddes, J.; O’Brien, G. A systematic review of the use of atypical antipsychotics in autism. J. Psychopharmacol. 2002, 16, 93–101. [Google Scholar] [CrossRef]
- Doyle, C.A.; McDougle, C.J. Pharmacotherapy to control behavioral symptoms in children with autism. Expert Opin. Pharmacother. 2012, 13, 1615–1629. [Google Scholar] [CrossRef] [PubMed]
- McDougle, C.J.; Stigler, K.A.; Erickson, C.A.; Posey, D.J. Pharmacology of Autism. Clin. Neurosci. Res. 2006, 6, 179–188. [Google Scholar] [CrossRef]
- Marcus, R.N.; Owen, R.; Kamen, L.; Manos, G.; McQuade, R.D.; Carson, W.H.; Aman, M.G. A Placebo-Controlled, Fixed-Dose Study of Aripiprazole in Children and Adolescents with Irritability Associated With Autistic Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2009, 48, 1110–1119. [Google Scholar] [CrossRef]
- McCracken, J.T.; McGough, J.; Shah, B.; Cronin, P.; Hong, D.; Aman, M.G.; Arnold, L.E.; Lindsay, R.; Nash, P.; Hollway, J.; et al. Risperidone in Children with Autism and Serious Behavioral Problems. N. Engl. J. Med. 2002, 347, 314–321. [Google Scholar] [CrossRef]
- Nicolson, R.; Awad, D.; Sloman, L. An Open Trial of Risperidone in Young Autistic Children. J. Am. Acad. Child Adolesc. Psychiatry 1998, 37, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Findling, R.L.; Maxwell, K.; Wiznitzer, M. An Open Clinical Trial of Risperidone Monotherapy in Young Children with Autistic Disorder. Psychopharmacol Bull. 1997, 33, 155–159. [Google Scholar] [PubMed]
- Mcdougle, C.J.; Holmes, J.P.; Bronson, M.R.; Anderson, G.M.; Volkmar, F.R.; Price, L.H.; Cohen, D.J. Risperidone Treatment of Children and Adolescents with Pervasive Developmental Disorders: A Prospective, Open-Label Study. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 685–693. [Google Scholar] [CrossRef]
- Mukaddes, N.M.; Abali, O.; Gurkan, K. Short-Term Efficacy and Safety of Risperidone in Young Children with Autistic Disorder (AD). World J. Biol. Psychiatry 2004, 5, 211–214. [Google Scholar] [CrossRef]
- Diler, R.S.; Firat, S.; Avci, A. An open-label trial of risperidone in children with autism. Curr. Ther. Res. 2002, 63, 91–102. [Google Scholar] [CrossRef]
- Hardan, A.; Johnson, K.; Johnson, C.; Hrecznyj, B. Case Study: Risperidone Treatment of Children and Adolescents with Developmental Disorders. J. Am. Acad. Child Adolesc. Psychiatry 1996, 35, 1551–1556. [Google Scholar] [CrossRef]
- Danial, J.T.; Wood, J.J. Cognitive Behavioral Therapy for Children with Autism: Review and Considerations for Future Research. J. Dev. Behav. Pediatr. 2013, 34, 702–715. [Google Scholar] [CrossRef]
- Roane, H.S.; Fisher, W.W.; Carr, J.E. Applied Behavior Analysis as Treatment for Autism Spectrum Disorder. J. Pediatr. 2016, 175, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Ventola, P. Pivotal response treatment for autism spectrum disorder: Current perspectives. Neuropsychiatr. Dis. Treat. 2017, 13, 1613–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, A.; Le Couteur, A.; Gottesman, I.; Bolton, P.; Simonoff, E.; Yuzda, E.; Rutter, M. Autism as a strongly genetic disorder: Evidence from a British twin study. Psychol. Med. 1995, 25, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Lauritsen, M.B.; Ewald, H. The Genetics of Autism. Acta Psychiatr. Scand. 2001, 103, 411–427. [Google Scholar] [CrossRef] [PubMed]
- Sandin, S.; Lichtenstein, P.; Kuja-Halkola, R.; Larsson, H.; Hultman, C.M.; Reichenberg, A. The Familial Risk of Autism. JAMA 2014, 311, 1770–1777. [Google Scholar] [CrossRef]
- Samsam, M.; Ahangari, R.; Naser, S.A. Pathophysiology of autism spectrum disorders: Revisiting gastrointestinal involvement and immune imbalance. World J. Gastroenterol. 2014, 20, 9942–9951. [Google Scholar] [CrossRef]
- Masini, E.; Loi, E.; Vega-Benedetti, A.F.; Carta, M.; Doneddu, G.; Fadda, R.; Zavattari, P. An Overview of the Main Genetic, Epigenetic and Environmental Factors Involved in Autism Spectrum Disorder Focusing on Synaptic Activity. Int. J. Mol. Sci. 2020, 21, 8290. [Google Scholar] [CrossRef]
- Eissa, N.; Al-Houqani, M.; Sadeq, A.; Ojha, S.K.; Sasse, A.; Sadek, B. Current Enlightenment About Etiology and Pharmacological Treatment of Autism Spectrum Disorder. Front. Neurosci. 2018, 12, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, S.H.; Choi, J.; Lee, W.J.; Do, J.T. Genetic and Epigenetic Etiology Underlying Autism Spectrum Disorder. J. Clin. Med. 2020, 9, 966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebat, J.; Lakshmi, B.; Malhotra, D.; Troge, J.; Lese-Martin, C.; Walsh, T.; Yamrom, B.; Yoon, S.; Krasnitz, A.; Kendall, J.; et al. Strong Association of De Novo Copy Number Mutations with Autism. Science 2007, 316, 445–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikhail, F.M.; Lose, E.J.; Robin, N.H.; Descartes, M.D.; Rutledge, K.D.; Rutledge, S.L.; Korf, B.R.; Carroll, A.J. Clinically Relevant Single Gene or Intragenic Deletions Encompassing Critical Neurodevelopmental Genes in Patients with Developmental Delay, Mental Retardation, and/or Autism Spectrum Disorders. Am. J. Med. Genet. A 2011, 155A, 2386–2396. [Google Scholar] [CrossRef] [PubMed]
- Iossifov, I.; Ronemus, M.; Levy, D.; Wang, Z.; Hakker, I.; Rosenbaum, J.; Yamrom, B.; Lee, Y.-H.; Narzisi, G.; Leotta, A.; et al. De Novo Gene Disruptions in Children on the Autistic Spectrum. Neuron 2012, 74, 285–299. [Google Scholar] [CrossRef] [Green Version]
- Iossifov, I.; O’Roak, B.J.; Sanders, S.J.; Ronemus, M.; Krumm, N.; Levy, D.; Stessman, H.A.; Witherspoon, K.T.; Vives, L.; Patterson, K.E.; et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 2014, 515, 216–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bölte, S.; Girdler, S.; Marschik, P.B. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell. Mol. Life Sci. 2019, 76, 1275–1297. [Google Scholar] [CrossRef] [Green Version]
- Karimi, P.; Kamali, E.; Mousavi, S.M.; Karahmadi, M. Environmental factors influencing the risk of autism. J. Res. Med. Sci. 2017, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Chugani, D.C. Role of altered brain serotonin mechanisms in autism. Mol. Psychiatry 2002, 7, S16–S17. [Google Scholar] [CrossRef] [Green Version]
- Cook, E.H.; Leventhal, B.L. The serotonin system in autism. Curr. Opin. Pediatr. 1996, 8, 348–354. [Google Scholar] [CrossRef]
- Muller, C.L.; Anacker, A.M.J.; Veenstra-VanderWeele, J. The serotonin system in autism spectrum disorder: From biomarker to animal models. Neuroscience 2016, 321, 24–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacivita, E.; Niso, M.; Mastromarino, M.; Silva, A.G.; Resch, C.; Zeug, A.; Loza, M.I.; Castro, M.; Ponimaskin, E.; Leopoldo, M. Knowledge-Based Design of Long-Chain Arylpiperazine Derivatives Targeting Multiple Serotonin Receptors as Potential Candidates for Treatment of Autism Spectrum Disorder. ACS Chem. Neurosci. 2021, 12, 1313–1327. [Google Scholar] [CrossRef]
- Blankenship, K.; Erickson, C.A.; Stigler, K.A.; Posey, D.J.; McDougle, C.J. Aripiprazole for irritability associated with autistic disorder in children and adolescents aged 6–17 years. Pediatr. Health 2010, 4, 375–381. [Google Scholar] [CrossRef]
- Cookson, J.; Pimm, J. Partial Agonists of Dopamine Receptors: Mechanisms and Clinical Effects of Aripiprazole, Brexpiprazole and Cariprazine. BJPsych Adv. 2021, 1–6. [Google Scholar] [CrossRef]
- Owen, R.; Sikich, L.; Marcus, R.N.; Corey-Lisle, P.; Manos, G.; McQuade, R.D.; Carson, W.H.; Findling, R.L. Aripiprazole in the Treatment of Irritability in Children and Adolescents with Autistic Disorder. Pediatrics 2009, 124, 1533–1540. [Google Scholar] [CrossRef] [Green Version]
- Risperidone Treatment of Autistic Disorder: Longer-Term Benefits and Blinded Discontinuation after 6 Months. Am. J. Psychiatry 2005, 162, 1361–1369. [CrossRef] [PubMed] [Green Version]
- Kent, J.M.; Kushner, S.; Ning, X.; Karcher, K.; Ness, S.; Aman, M.; Singh, J.; Hough, D. Risperidone Dosing in Children and Adolescents with Autistic Disorder: A Double-Blind, Placebo-Controlled Study. J. Autism Dev. Disord. 2013, 43, 1773–1783. [Google Scholar] [CrossRef]
- Malone, R.P.; Maislin, G.; Choudhury, M.S.; Gifford, C.; Delaney, M.A. Risperidone Treatment in Children and Adolescents with Autism: Short- and Long-Term Safety and Effectiveness. J. Am. Acad. Child Adolesc. Psychiatry 2002, 41, 140–147. [Google Scholar] [CrossRef]
- Canitano, R. Self injurious behavior in autism: Clinical aspects and treatment with risperidone. J. Neural Transm. 2006, 113, 425–431. [Google Scholar] [CrossRef]
- Loebel, A.; Brams, M.; Goldman, R.S.; Silva, R.; Hernandez, D.; Deng, L.; Mankoski, R.; Findling, R.L. Lurasidone for the Treatment of Irritability Associated with Autistic Disorder. J. Autism Dev. Disord. 2016, 46, 1153–1163. [Google Scholar] [CrossRef] [Green Version]
- Witt, N.A.; Lee, B.; Ghent, K.; Zhang, W.Q.; Pehrson, A.L.; Sánchez, C.; Gould, G.G. Vortioxetine Reduces Marble Burying but Only Transiently Enhances Social Interaction Preference in Adult Male BTBR T+Itpr3tf/J Mice. ACS Chem. Neurosci. 2019, 10, 4319–4327. [Google Scholar] [CrossRef]
- Gordon, C.T.; State, R.C.; Nelson, J.E.; Hamburger, S.D.; Rapoport, J.L. A Double-blind Comparison of Clomipramine, Desipramine, and Placebo in the Treatment of Autistic Disorder. Arch. Gen. Psychiatry 1993, 50, 441–447. [Google Scholar] [CrossRef]
- Hollander, E.; Phillips, A.; Chaplin, W.; Zagursky, K.; Novotny, S.; Wasserman, S.; Iyengar, R. A Placebo Controlled Crossover Trial of Liquid Fluoxetine on Repetitive Behaviors in Childhood and Adolescent Autism. Neuropsychopharmacology 2005, 30, 582–589. [Google Scholar] [CrossRef] [Green Version]
- McDougle, C.J.; Naylor, S.T.; Cohen, D.J.; Volkmar, F.R.; Heninger, G.R.; Price, L.H. A Double-blind, Placebo-Controlled Study of Fluvoxamine in Adults with Autistic Disorder. Arch. Gen. Psychiatry 1996, 53, 1001–1008. [Google Scholar] [CrossRef]
- Delong, G.R.; Teague, L.A.; Kamran, M.M. Effects of fluoxetine treatment in young children with idiopathic autism. Dev. Med. Child Neurol. 2008, 40, 551–562. [Google Scholar] [CrossRef] [Green Version]
- McDougle, C.J.; Brodkin, E.S.; Naylor, S.T.; Carlson, D.C.; Cohen, D.J.; Price, L.H. Sertraline in Adults with Pervasive Developmental Disorders: A Prospective Open-Label Investigation. J. Clin. Psychopharmacol. 1998, 18, 62–66. [Google Scholar] [CrossRef]
- Steingard, R.J.; Zimnitzky, B.; DeMaso, D.R.; Bauman, M.L.; Bucci, J.P. Sertraline Treatment of Transition-Associated Anxiety and Agitation in Children with Autistic Disorder. J. Child Adolesc. Psychopharmacol. 1997, 7, 9–15. [Google Scholar] [CrossRef]
- Sugie, Y.; Sugie, H.; Fukuda, T.; Ito, M.; Sasada, Y.; Nakabayashi, M.; Fukashiro, K.; Ohzeki, T. Clinical Efficacy of Fluvoxamine and Functional Polymorphism in a Serotonin Transporter Gene on Childhood Autism. J. Autism Dev. Disord. 2005, 35, 377–385. [Google Scholar] [CrossRef]
- Canal, C.E.; Felsing, D.E.; Liu, Y.; Zhu, W.; Wood, J.T.; Perry, C.K.; Vemula, R.; Booth, R.G. An Orally Active Phenylaminotetralin-Chemotype Serotonin 5-Ht7 and 5-Ht1a Receptor Partial Agonist That Corrects Motor Stereotypy in Mouse Models. ACS Chem. Neurosci. 2015, 6, 1259–1270. [Google Scholar] [CrossRef]
- Armstrong, J.L.; Casey, A.B.; Saraf, T.S.; Mukherjee, M.; Booth, R.G.; Canal, C.E. (S)-5-(2′-Fluorophenyl)-N,N-Dimethyl-1,2,3,4-Tetrahydronaphthalen-2-Amine, a Serotonin Receptor Modulator, Possesses Anticonvulsant, Prosocial, and Anxiolytic-Like Properties in an Fmr1 Knockout Mouse Model of Fragile X Syndrome and Autism Spectrum Disorder. ACS Pharmacol. Transl. Sci. 2020, 3, 509–523. [Google Scholar]
- Wu, H.-F.; Chen, Y.-J.; Chu, M.-C.; Hsu, Y.-T.; Lu, T.-Y.; Chen, I.-T.; Chen, P.S.; Lin, H.-C. Deep Brain Stimulation Modified Autism-Like Deficits via the Serotonin System in a Valproic Acid-Induced Rat Model. Int. J. Mol. Sci. 2018, 19, 2840. [Google Scholar] [CrossRef] [Green Version]
- Kwag, R.; Lee, J.; Kim, D.; Lee, H.; Yeom, M.; Woo, J.; Cho, Y.; Kim, H.J.; Kim, J.; Keum, G.; et al. Discovery of G Protein-Biased Antagonists against 5-HT7R. J. Med. Chem. 2021, 64, 13766–13779. [Google Scholar] [CrossRef]
- Nisar, S.; Bhat, A.A.; Masoodi, T.; Hashem, S.; Akhtar, S.; Ali, T.A.; Amjad, S.; Chawla, S.; Bagga, P.; Frenneaux, M.P.; et al. Genetics of glutamate and its receptors in autism spectrum disorder. Mol. Psychiatry 2022, 27, 2380–2392. [Google Scholar] [CrossRef]
- Choudhury, P.R.; Lahiri, S.; Rajamma, U. Glutamate mediated signaling in the pathophysiology of autism spectrum disorders. Pharmacol. Biochem. Behav. 2012, 100, 841–849. [Google Scholar] [CrossRef]
- Aldred, S.; Moore, K.M.; Fitzgerald, M.; Waring, R.H. Plasma amino acid levels in children with autism and their families. J. Autism Dev. Disord. 2003, 33, 93–97. [Google Scholar] [CrossRef]
- Shinohe, A.; Hashimoto, K.; Nakamura, K.; Tsujii, M.; Iwata, Y.; Tsuchiya, K.J.; Sekine, Y.; Suda, S.; Suzuki, K.; Sugihara, G.-I.; et al. Increased Serum Levels of Glutamate in Adult Patients with Autism. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 1472–1477. [Google Scholar] [CrossRef] [Green Version]
- Rojas, D.C. The role of glutamate and its receptors in autism and the use of glutamate receptor antagonists in treatment. J. Neural Transm. 2014, 121, 891–905. [Google Scholar] [CrossRef] [Green Version]
- Lenart, J.; Augustyniak, J.; Lazarewicz, J.W.; Zieminska, E. Altered expression of glutamatergic and GABAergic genes in the valproic acid-induced rat model of autism: A screening test. Toxicology 2020, 440, 152500. [Google Scholar] [CrossRef]
- Carlson, G.C. Glutamate receptor dysfunction and drug targets across models of autism spectrum disorders. Pharmacol. Biochem. Behav. 2012, 100, 850–854. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Hou, F.; Li, L.; Chen, Y.; Liu, L.; Luo, X.; Gu, H.; Li, X.; Zhang, J.; Gong, J.; et al. Polymorphisms of Ionotropic Glutamate Receptor-Related Genes and the Risk of Autism Spectrum Disorder in a Chinese Population. Psychiatry Investig. 2019, 16, 379–385. [Google Scholar] [CrossRef]
- Jamain, S.; Betancur, C.; Quach, H.; Philippe, A.; Fellous, M.; Giros, B.; Gillberg, C.; Leboyer, M.; Bourgeron, T.; The Paris Autism Research International Sibpair (PARIS) Study. Linkage and association of the glutamate receptor 6 gene with autism. Mol. Psychiatry 2002, 7, 302–310. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-W.; Park, K.; Kang, R.J.; Gonzales, E.L.T.; Kim, D.G.; Oh, H.A.; Seung, H.; Ko, M.J.; Kwon, K.J.; Kim, K.C.; et al. Pharmacological modulation of AMPA receptor rescues social impairments in animal models of autism. Neuropsychopharmacology 2019, 44, 314–323. [Google Scholar] [CrossRef] [Green Version]
- Silverman, J.L.; Oliver, C.F.; Karras, M.N.; Gastrell, P.T.; Crawley, J.N. AMPAKINE enhancement of social interaction in the BTBR mouse model of autism. Neuropharmacology 2013, 64, 268–282. [Google Scholar] [CrossRef] [Green Version]
- Sacai, H.; Sakoori, K.; Konno, K.; Nagahama, K.; Suzuki, H.; Watanabe, T.; Watanabe, M.; Uesaka, N.; Kano, M. Autism spectrum disorder-like behavior caused by reduced excitatory synaptic transmission in pyramidal neurons of mouse prefrontal cortex. Nat. Commun. 2020, 11, 5140. [Google Scholar] [CrossRef]
- Chez, M.G.; Burton, Q.; Dowling, T.; Chang, M.; Khanna, P.; Kramer, C. Memantine as Adjunctive Therapy in Children Diagnosed with Autistic Spectrum Disorders: An Observation of Initial Clinical Response and Maintenance Tolerability. J. Child Neurol. 2007, 22, 574–579. [Google Scholar] [CrossRef]
- Karahmadi, M.; Tarrahi, M.J.; Ardestani, S.S.V.; Omranifard, V.; Farzaneh, B. Efficacy of Memantine as Adjunct Therapy for Autism Spectrum Disorder in Children Aged < 14 Years. Adv. Biomed. Res. 2018, 7, 131. [Google Scholar] [CrossRef]
- Joshi, G.; Wozniak, J.; Faraone, S.V.; Fried, R.; Chan, J.; Furtak, S.; Grimsley, E.; Conroy, K.; Kilcullen, J.R.; Woodworth, K.Y.; et al. A Prospective Open-Label Trial of Memantine Hydrochloride for the Treatment of Social Deficits in Intellectually Capable Adults with Autism Spectrum Disorder. J. Clin. Psychopharmacol. 2016, 36, 262–271. [Google Scholar] [CrossRef]
- Deutsch, S.I.; Pepe, G.J.; Burket, J.A.; Winebarger, E.E.; Herndon, A.L.; Benson, A.D. D-cycloserine improves sociability and spontaneous stereotypic behaviors in 4-week old mice. Brain Res. 2012, 1439, 96–107. [Google Scholar] [CrossRef]
- Modi, M.E.; Young, L.J. D-Cycloserine Facilitates Socially Reinforced Learning in an Animal Model Relevant to Autism Spectrum Disorders. Biol. Psychiatry 2011, 70, 298–304. [Google Scholar] [CrossRef] [Green Version]
- Posey, D.J.; Kem, D.L.; Swiezy, N.B.; Sweeten, T.L.; Wiegand, R.E.; McDougle, C.J. A Pilot Study of d-Cycloserine in Subjects with Autistic Disorder. Am. J. Psychiatry 2004, 161, 2115–2117. [Google Scholar] [CrossRef]
- Urbano, M.; Okwara, L.; Manser, P.; Hartmann, K.; Herndon, A.; Deutsch, S.I. A Trial of D-Cycloserine to Treat Stereotypies in Older Adolescents and Young Adults with Autism Spectrum Disorder. Clin. Neuropharmacol. 2014, 37, 69–72. [Google Scholar] [CrossRef] [Green Version]
- Wink, L.K.; Minshawi, N.F.; Shaffer, R.C.; Plawecki, M.H.; Posey, D.J.; Horn, P.S.; Adams, R.; Pedapati, E.V.; Schaefer, T.L.; McDougle, C.J.; et al. D-Cycloserine enhances durability of social skills training in autism spectrum disorder. Mol. Autism 2017, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Zoicas, I.; Kornhuber, J. The Role of Metabotropic Glutamate Receptors in Social Behavior in Rodents. Int. J. Mol. Sci. 2019, 20, 1412. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.H.; Lee, J.; Lee, J.-Y.; Roche, K.W. Metabotropic glutamate receptors: Phosphorylation and receptor signaling. J. Neurosci. Res. 2007, 86, 1–10. [Google Scholar] [CrossRef]
- Aguilar-Valles, A.; Matta-Camacho, E.; Khoutorsky, A.; Gkogkas, C.; Nader, K.; Lacaille, J.-C.; Sonenberg, N. Inhibition of Group I Metabotropic Glutamate Receptors Reverses Autistic-Like Phenotypes Caused by Deficiency of the Translation Repressor eIF4E Binding Protein 2. J. Neurosci. 2015, 35, 11125–11132. [Google Scholar] [CrossRef] [Green Version]
- Silverman, J.L.; Tolu, S.S.; Barkan, C.L.; Crawley, J.N. Repetitive Self-Grooming Behavior in the BTBR Mouse Model of Autism is Blocked by the mGluR5 Antagonist MPEP. Neuropsychopharmacology 2010, 35, 976–989. [Google Scholar] [CrossRef]
- Mehta, M.V.; Gandal, M.J.; Siegel, S.J. Mglur5-Antagonist Mediated Reversal of Elevated Stereotyped, Repetitive Behaviors in the Vpa Model of Autism. PLoS ONE 2011, 6, e26077. [Google Scholar] [CrossRef] [Green Version]
- Erickson, C.A.; Early, M.; Stigler, K.A.; Wink, L.K.; Mullett, J.E.; McDougle, C.J. An Open-Label Naturalistic Pilot Study of Acamprosate in Youth with Autistic Disorder. J. Child. Adolesc. Psychopharmacol. 2011, 21, 565–569. [Google Scholar] [CrossRef] [Green Version]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Ho, L.K.H.; Tong, V.J.W.; Syn, N.; Nagarajan, N.; Tham, E.H.; Tay, S.K.; Shorey, S.; Tambyah, P.A.; Law, E.C.N. Gut microbiota changes in children with autism spectrum disorder: A systematic review. Gut Pathog. 2020, 12, 6. [Google Scholar] [CrossRef] [Green Version]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef] [Green Version]
- Luk, B.; Veeraragavan, S.; Engevik, M.; Balderas, M.; Major, A.; Runge, J.; Luna, R.A.; Versalovic, J. Postnatal colonization with human “infant-type” Bifidobacterium species alters behavior of adult gnotobiotic mice. PLoS ONE 2018, 13, e0196510. [Google Scholar] [CrossRef]
- Zheng, Y.; Bek, M.K.; Prince, N.Z.; Marzal, L.N.P.; Garssen, J.; Pardo, P.P.; Kraneveld, A.D. The Role of Bacterial-Derived Aromatic Amino Acids Metabolites Relevant in Autism Spectrum Disorders: A Comprehensive Review. Front. Neurosci. 2021, 15, 738220. [Google Scholar] [CrossRef]
- Needham, B.D.; Adame, M.D.; Serena, G.; Rose, D.R.; Preston, G.M.; Conrad, M.C.; Campbell, A.S.; Donabedian, D.H.; Fasano, A.; Ashwood, P.; et al. Plasma and Fecal Metabolite Profiles in Autism Spectrum Disorder. Biol. Psychiatry 2020, 89, 451–462. [Google Scholar] [CrossRef]
- Mead, J.; Ashwood, P. Evidence supporting an altered immune response in ASD. Immunol. Lett. 2015, 163, 49–55. [Google Scholar] [CrossRef]
- Chez, M.G.; Guido-Estrada, N. Immune therapy in autism: Historical experience and future directions with immunomodulatory therapy. Neurotherapeutics 2010, 7, 293–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masi, A.; Quintana, D.S.; Glozier, N.; Lloyd, A.R.; Hickie, I.B.; Guastella, A.J. Cytokine aberrations in autism spectrum disorder: A systematic review and meta-analysis. Mol. Psychiatry 2015, 20, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Marchezan, J.; Dos Santos, E.G.A.W.; Deckmann, I.; Riesgo, R.S. Immunological Dysfunction in Autism Spectrum Disorder: A Potential Target for Therapy. Neuroimmunomodulation 2018, 25, 300–319. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.; Glozier, N.; Dale, R.; Guastella, A.J. The Immune System, Cytokines, and Biomarkers in Autism Spectrum Disorder. Neurosci. Bull. 2017, 33, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Nordahl, C.W.; Braunschweig, D.; Iosif, A.-M.; Lee, A.; Rogers, S.; Ashwood, P.; Amaral, D.G.; Van de Water, J. Maternal autoantibodies are associated with abnormal brain enlargement in a subgroup of children with autism spectrum disorder. Brain Behav. Immun. 2013, 30, 61–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker-Athill, E.C.; Tan, J. Maternal Immune Activation and Autism Spectrum Disorder: Interleukin-6 Signaling as a Key Mechanistic Pathway. Neurosignals 2010, 18, 113–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atladóttir, H.Ó.; Thorsen, P.; Østergaard, L.; Schendel, D.E.; Lemcke, S.; Abdallah, M.; Partner, E.T. Maternal Infection Requiring Hospitalization During Pregnancy and Autism Spectrum Disorders. J. Autism Dev. Disord. 2010, 40, 1423–1430. [Google Scholar] [CrossRef]
- Brown, A.S.; Sourander, A.; Hinkka-Yli-Salomäki, S.; McKeague, I.W.; Sundvall, J.; Surcel, H.-M. Elevated maternal C-reactive protein and autism in a national birth cohort. Mol. Psychiatry 2014, 19, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Lammert, C.R.; Lukens, J.R. Modeling Autism-Related Disorders in Mice with Maternal Immune Activation (MIA). Methods Mol. Biol. 2019, 1960, 227–236. [Google Scholar] [CrossRef]
- Yim, Y.S.; Park, A.; Berrios, J.; Lafourcade, M.; Pascual, L.M.; Soares, N.; Kim, J.Y.; Kim, S.; Kim, H.; Waisman, A.; et al. Reversing behavioural abnormalities in mice exposed to maternal inflammation. Nature 2017, 549, 482–487. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Kim, H.; Yim, Y.S.; Ha, S.; Atarashi, K.; Tan, T.G.; Longman, R.S.; Honda, K.; Littman, D.R.; Choi, G.B.; et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 2017, 549, 528–532. [Google Scholar] [CrossRef] [Green Version]
- Estes, M.L.; McAllister, A.K. Maternal Immune Activation: Implications for Neuropsychiatric Disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef] [Green Version]
- Lammert, C.R.; Frost, E.L.; Bolte, A.C.; Paysour, M.J.; Shaw, M.E.; Bellinger, C.E.; Weigel, T.K.; Zunder, E.R.; Lukens, J.R. Cutting Edge: Critical Roles for Microbiota-Mediated Regulation of the Immune System in a Prenatal Immune Activation Model of Autism. J. Immunol. 2018, 201, 845–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egottfried, C.; Bambini-Junior, V.; Francis, F.; Riesgo, R.; Savino, W. The Impact of Neuroimmune Alterations in Autism Spectrum Disorder. Front. Psychiatry 2015, 6, 121. [Google Scholar] [CrossRef] [Green Version]
- Dantzer, R. Cytokine-Induced Sickness Behavior: Where Do We Stand? Brain Behav. Immun. 2001, 15, 7–24. [Google Scholar] [CrossRef] [Green Version]
- Krakowiak, P.; Goines, P.E.; Tancredi, D.J.; Ashwood, P.; Hansen, R.L.; Hertz-Picciotto, I.; Van de Water, J. Neonatal Cytokine Profiles Associated with Autism Spectrum Disorder. Biol. Psychiatry 2015, 81, 442–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K.; Matsuzaki, H.; Iwata, K.; Kameno, Y.; Shimmura, C.; Kawai, S.; Yoshihara, Y.; Wakuda, T.; Takebayashi, K.; Takagai, S.; et al. Plasma Cytokine Profiles in Subjects with High-Functioning Autism Spectrum Disorders. PLoS ONE 2011, 6, e20470. [Google Scholar] [CrossRef]
- Ashwood, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Pessah, I.; Van de Water, J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 2011, 25, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Jácome, M.C.I.; Chacòn, L.M.M.; Cuesta, H.V.; Rizo, C.M.; Santiesteban, M.W.; Hernandez, L.R.; García, E.N.; Fraguela, M.E.G.; Verdecia, C.I.F.; Hurtado, Y.V.; et al. Peripheral Inflammatory Markers Contributing to Comorbidities in Autism. Behav. Sci. 2016, 6, 29. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Huang, L.; Li, X.; Li, H.; Zhou, Y.; Zhu, H.; Pan, T.; Kendrick, K.M.; Xu, W. Immunological cytokine profiling identifies TNF-α as a key molecule dysregulated in autistic children. Oncotarget 2017, 8, 82390–82398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Ayadhi, L.Y.; Mostafa, G.A. Elevated serum levels of interleukin-17A in children with autism. J. Neuroinflammation 2012, 9, 158. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.-C.; Xu, X.; Xiong, G.-L.; Xu, Q.; Zhou, B.-R.; Li, C.-Y.; Qin, Q.; Liu, C.-X.; Li, H.-P.; Sun, Y.-J.; et al. Alterations in plasma cytokine levels in chinese children with autism spectrum disorder. Autism Res. 2018, 11, 989–999. [Google Scholar] [CrossRef]
- Emanuele, E.; Orsi, P.; Boso, M.; Broglia, D.; Brondino, N.; Barale, F.; di Nemi, S.U.; Politi, P. Low-grade endotoxemia in patients with severe autism. Neurosci. Lett. 2010, 471, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Parker-Athill, E.; Luo, D.; Bailey, A.; Giunta, B.; Tian, J.; Shytle, R.D.; Murphy, T.; Legradi, G.; Tan, J. Flavonoids, a Prenatal Prophylaxis Via Targeting Jak2/Stat3 Signaling to Oppose Il-6/Mia Associated Autism. J. Neuroimmunol. 2009, 217, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Theoharides, T.C.; Asadi, S.; Panagiotidou, S. A Case Series of a Luteolin Formulation (Neuroprotek®) in Children with Autism Spectrum Disorders. Int. J. Immunopathol. Pharmacol. 2012, 25, 317–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taliou, A.; Zintzaras, E.; Lykouras, L.; Francis, K. An Open-Label Pilot Study of a Formulation Containing the Anti-Inflammatory Flavonoid Luteolin and Its Effects on Behavior in Children with Autism Spectrum Disorders. Clin. Ther. 2013, 35, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Kann, O.; Hoffmann, A.; Schumann, R.R.; Weber, J.R.; Kettenmann, H.; Hanisch, U.-K. The tyrosine kinase inhibitor AG126 restores receptor signaling and blocks release functions in activated microglia (brain macrophages) by preventing a chronic rise in the intracellular calcium level. J. Neurochem. 2004, 90, 513–525. [Google Scholar] [CrossRef]
- Menzfeld, C.; John, M.; Van Rossum, D.; Regen, T.; Scheffel, J.; Janova, H.; Götz, A.; Ribes, S.; Nau, R.; Borisch, A.; et al. Tyrphostin AG126 exerts neuroprotection in CNS inflammation by a dual mechanism. Glia 2015, 63, 1083–1099. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.F.; Ansari, M.A.; Nadeem, A.; Bakheet, S.A.; Alsanea, S.; Al-Hosaini, K.A.; Mohammad, H.M.; Alzahrani, M.Z.; Attia, S.M. Inhibition of Tyrosine Kinase Signaling by Tyrphostin Ag126 Downregulates the Il-21/Il-21r and Jak/Stat Pathway in the Btbr Mouse Model of Autism. NeuroToxicology 2020, 77, 1–11. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Ansari, M.A.; Nadeem, A.; Bakheet, S.A.; Alshammari, M.A.; Attia, S.A. Protection by Tyrosine Kinase Inhibitor, Tyrphostin Ag126, through the Suppression of Il-17a, Rorγt, and T-Bet Signaling, in the Btbr Mouse Model of Autism. Brain Res. Bull. 2018, 142, 328–337. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Ansari, M.A.; Nadeem, A.; Bakheet, S.A.; Alzahrani, M.Z.; Alshammari, M.A.; Alanazi, W.A.; Alasmari, A.F.; Attia, S.M. Resveratrol attenuates pro-inflammatory cytokines and activation of JAK1-STAT3 in BTBR T + Itpr3 tf /J autistic mice. Eur. J. Pharmacol. 2018, 829, 70–78. [Google Scholar] [CrossRef]
- Bakheet, S.A.; Alzahrani, M.Z.; Nadeem, A.; Ansari, M.A.; Zoheir, K.M.A.; Attia, S.M.; Al-Ayadhi, L.Y.; Ahmad, S.F. Resveratrol treatment attenuates chemokine receptor expression in the BTBR T + tf/J mouse model of autism. Mol. Cell. Neurosci. 2016, 77, 1–10. [Google Scholar] [CrossRef]
- Bambini-Junior, V.; Zanatta, G.; Nunes, G.D.F.; de Melo, G.M.; Michels, M.; Fontes-Dutra, M.; Freire, V.N.; Riesgo, R.; Gottfried, C. Resveratrol prevents social deficits in animal model of autism induced by valproic acid. Neurosci. Lett. 2014, 583, 176–181. [Google Scholar] [CrossRef]
- Bakheet, S.A.; Alzahrani, M.Z.; Ansari, M.A.; Nadeem, A.; Zoheir, K.M.A.; Attia, S.M.; Al-Ayadhi, L.Y.; Ahmad, S.F. Resveratrol Ameliorates Dysregulation of Th1, Th2, Th17, and T Regulatory Cell-Related Transcription Factor Signaling in a BTBR T + tf/J Mouse Model of Autism. Mol. Neurobiol. 2017, 54, 5201–5212. [Google Scholar] [CrossRef]
- Hidema, S.; Kikuchi, S.; Takata, R.; Yanai, T.; Shimomura, K.; Horie, K.; Nishimori, K. Single administration of resveratrol improves social behavior in adult mouse models of autism spectrum disorder. Biosci. Biotechnol. Biochem. 2020, 84, 2207–2214. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Khan, H.; Cauli, O. Resveratrol in Autism Spectrum Disorders: Behavioral and Molecular Effects. Antioxidants 2020, 9, 188. [Google Scholar] [CrossRef] [Green Version]
- Hendouei, F.; Moghaddam, H.S.; Mohammadi, M.R.; Taslimi, N.; Rezaei, F.; Akhondzadeh, S. Resveratrol as adjunctive therapy in treatment of irritability in children with autism: A double-blind and placebo-controlled randomized trial. J. Clin. Pharm. Ther. 2020, 45, 324–334. [Google Scholar] [CrossRef]
- McDougle, C.J.; Landino, S.M.; Vahabzadeh, A.; O’Rourke, J.; Zurcher, N.R.; Finger, B.C.; Palumbo, M.L.; Helt, J.; Mullett, J.E.; Hooker, J.M.; et al. Toward an Immune-Mediated Subtype of Autism Spectrum Disorder. Brain Res. 2015, 1617, 72–92. [Google Scholar] [CrossRef]
- Asadabadi, M.; Mohammadi, M.-R.; Ghanizadeh, A.; Modabbernia, A.; Ashrafi, M.; Hassanzadeh, E.; Forghani, S.; Akhondzadeh, S. Celecoxib as Adjunctive Treatment to Risperidone in Children with Autistic Disorder: A Randomized, Double-Blind, Placebo-Controlled Trial. Psychopharmacology 2013, 225, 51–59. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Fallah, J.; Mohammadi, M.-R.; Imani, R.; Mohammadi, M.; Salehi, B.; Ghanizadeh, A.; Raznahan, M.; Mohebbi-Rasa, S.; Rezazadeh, S.-A.; et al. Double-blind placebo-controlled trial of pentoxifylline added to risperidone: Effects on aberrant behavior in children with autism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 32–36. [Google Scholar] [CrossRef]
- Dunn, P.M.; Blakeley, A.G.H. Suramin: A reversible P2-purinoceptor antagonist in the mouse vas deferens. Br. J. Pharmacol. 1988, 93, 243–245. [Google Scholar] [CrossRef] [Green Version]
- Herrera, A.; Morcuende, S.; Talaverón, R.; Benítez-Temiño, B.; Pastor, A.M.; Matarredona, E.R. Purinergic Receptor Blockade with Suramin Increases Survival of Postnatal Neural Progenitor Cells In Vitro. Int. J. Mol. Sci. 2021, 22, 713. [Google Scholar] [CrossRef]
- Hirsch, M.M.; Deckmann, I.; Santos-Terra, J.; Staevie, G.Z.; Fontes-Dutra, M.; Carello-Collar, G.; Körbes-Rockenbach, M.; Schwingel, G.B.; Bauer-Negrini, G.; Rabelo, B.; et al. Effects of single-dose antipurinergic therapy on behavioral and molecular alterations in the valproic acid-induced animal model of autism. Neuropharmacology 2020, 167, 107930. [Google Scholar] [CrossRef]
- Naviaux, J.C.; Schuchbauer, M.A.; Li, K.; Wang, L.; Risbrough, V.B.; Powell, S.B.; Naviaux, R.K. Reversal of autism-like behaviors and metabolism in adult mice with single-dose antipurinergic therapy. Transl. Psychiatry 2014, 4, e400. [Google Scholar] [CrossRef]
- Naviaux, R.K.; Zolkipli, Z.; Wang, L.; Nakayama, T.; Naviaux, J.C.; Le, T.P.; Schuchbauer, M.A.; Rogac, M.; Tang, Q.; Dugan, L.L.; et al. Antipurinergic Therapy Corrects the Autism-Like Features in the Poly(IC) Mouse Model. PLoS ONE 2013, 8, e57380. [Google Scholar] [CrossRef] [PubMed]
- Naviaux, R.K.; Curtis, B.; Li, K.; Naviaux, J.C.; Bright, A.T.; Reiner, G.E.; Westerfield, M.; Goh, S.; Alaynick, W.A.; Wang, L.; et al. Low-Dose Suramin in Autism Spectrum Disorder: A Small, Phase I/Ii, Randomized Clinical Trial. Ann. Clin. Transl. Neurol. 2017, 4, 491–505. [Google Scholar] [CrossRef] [PubMed]



| Compounds | Structures | Targets | Stage | Effects |
|---|---|---|---|---|
| Aripiprazole |  | Partial 5-HT1AR/5-HT2CR, agonist, 5-HT1BR/5-HT1DR/5-HT2AR/5-HT2CR/5-HT3AR/5-HT6R/5-HT7R antagonist | Clinical | Alleviating irritability [16,44,46] |
| Risperidone |  | 5-HT1AR/5-HT1DR/5-HT2AR/5-HT2CR/5-HT7R antagonist | Clinical | Effective in irritability, aggression, temper-outburst and self-injurious behavior [47,48,49] |
| Lurasidone |  | 5-HT2AR/5-HT7R antagonist, 5-HT1AR partial agonist | Clinical | Alleviating irritability [51] |
| Clomipramine |  | Potent SSRI | Clinical | Alleviating obsessive-compulsive disorder and abnormal social interaction [53] |
| Vortioxetine |  | 5-HT transporter, 5-HT1A/5-HT1B activator | Preclinical | Reduced repetitive behavior [52] |
| Fluoxetine |  | SSRI | Clinical | Improvement in repetitive behavior, social interactions, language and cognition [54,56] |
| Fluvoxamine |  | SSRI | Clinical | Improvement in repetitive behavior, maladaptive behavior, aggression, social interaction and language usage [55,59] |
| Sertraline |  | SSRI | Clinical | Improvement in repetitive behavior, aggression, anxiety, irritability and agitation [57,58] |
| (+)-5-FPT |  | 5-HT1AR/5-HT2CR agonist, 5-HT7R antagonist | Preclinical | Reduced repetitive behavior [60,61] |
| 8-OH DPAT |  | 5-HT1AR/5-HT7R agonist | Preclinical | Improvement in social interaction, anxiety and hyperactivity [62] |
| 2c |  | 5-HT7R antagonist | Preclinical | Reduced repetitive behavior [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.; Lee, S. Chemical Modulators for Targeting Autism Spectrum Disorders: From Bench to Clinic. Molecules 2022, 27, 5088. https://doi.org/10.3390/molecules27165088
Lim S, Lee S. Chemical Modulators for Targeting Autism Spectrum Disorders: From Bench to Clinic. Molecules. 2022; 27(16):5088. https://doi.org/10.3390/molecules27165088
Chicago/Turabian StyleLim, Songhyun, and Sanghee Lee. 2022. "Chemical Modulators for Targeting Autism Spectrum Disorders: From Bench to Clinic" Molecules 27, no. 16: 5088. https://doi.org/10.3390/molecules27165088
APA StyleLim, S., & Lee, S. (2022). Chemical Modulators for Targeting Autism Spectrum Disorders: From Bench to Clinic. Molecules, 27(16), 5088. https://doi.org/10.3390/molecules27165088