Silver Nanoparticles and Its Mechanistic Insight for Chronic Wound Healing: Review on Recent Progress
Abstract
:1. Introduction
2. Wound Infection Development and Its Progression
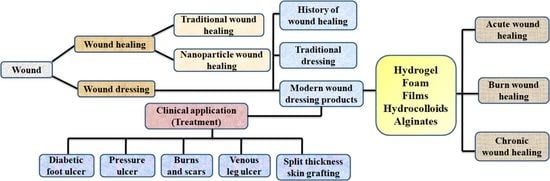
3. Traditional Wound Healing Approach
4. Nanotechnology and Wound Healing
5. Mechanistic Insight into Silver Nanoparticles (AgNPs)
6. AgNPs Associated Wound Dressings
6.1. AgNPs as Nanocomposite Material
6.2. AgNPs as Nanofibers
6.3. AgNPs as Hydrogels
6.4. AgNPs in Semi-Permeable Film Dressings
| Wound Dressing Materials | Size of AgNPs (nm) | Microorganism | In Vivo/In Vitro Model | Advantage of Nanocoating | Reference |
|---|---|---|---|---|---|
| Polycaprolactone/Gelatin (PCLGelAg) | 9–15 | S. aureus and P. aeruginosa | Mice model | Membrane coated dressings revealed more significant antibacterial activities compared to single coating. | [92] |
| Chitosan/Poly(Ethylene Oxide) matrix | 5 | E. coli | - | Introduction of AgNPs enhanced the antibacterial activity based on their shape and size | [122] |
| Chitosan-Poly Vinyl Pyrrolidone (PVP) composite | 10–30 | E. coli and S. aureus | 1929 cell line | Silver nanocomposite reduced the number of inflammatory cells by 99 in comparison to the control sample | [123] |
| Silver Alginate/Nicotinamide Nanocomposites | 20–80 | E. coli and S. aureus | Mice | Wound healing was achieved significantly after 4th day of treatment | [124] |
| Silver-Chitosan NPs-L-Glutamic Acid/Hyaluronic Acid | 5–30 | E. coli and S. aureus | Rabbit | Nanoparticle based natural matrix showed less inflammation in wounds compared with control after 15 days. | [125] |
| Cellulose hydrogel | 5–50 | E. coli and S. aureus | New Zealand rabbit | The average time for wound healing was 3 days in advance nanohydrogel compared to the control | [126] |
| Chitosan nanofiber | 25 | S. aureus | Wistar Hannover rats | The release of silver was significantly influenced by biological media: proteins created a barrier to silver release, whereas inorganic ions caused a sluggish release. As a result, inclusion of a large number of Ag-NPs was necessary to produce in vivo antibacterial effects. | [127] |
| Silver NPs embedded Bacterial cellulose gel membranes | 30 | S. aureus | Westar rats | After fourteen days of treatment, the wound healed (85.92%) significantly. | [128] |
| Chitosan-based multifunctional hydrogel | 250 | E. coli and S. aureus | Rat model | After 14 of treatment, the test organism exhibited lowest re-epithelialization rate | [129] |
| Chitosan-PEG hydrogel | 75 | E. coli, P. aeruginosa and S. aureus | Rabbit | At day fourteen, the Ag-NPs impregnated chitosan-PEG hydrogel group showed a healthy layer of dermal skin and a mixed pattern of collagen. | [115] |
| Chitosan cross-linked bilayer nanocomposite | 45 | E. coli, P. aeruginosa and S. aureus | L929 cell line | In comparison to the control group, the sustained-grown epithelium in the treatment group was more orderly and mature. | [130] |
| Asymmetric Wettable Chitosan nanocomposite | 25 | E. coli, P. aeruginosa and S. aureus | HEK293 cell line | An in vitro cytocompatibility investigation demonstrates that the dressing promotes cell development. | [131] |
| Polyvinyl-Pyrrolidone-Coated Silver Nanoparticles | 10 | E. coli and S. aureus | Mouse fibroblast (L929) cell line | Silver nanoparticles incorporated in PVP hydrogel led to cell enlargement. | [132] |
| Chitosan gels | 15 | P. aeruginosa | Human dermal fibroblasts | The evaluation of biocompatibility on primary fibroblasts revealed better results when the chitosan gels with Ag-NPs were analyzed | [133] |
| β-chitin-based hydrogels | 5 | E. coli and S. aureus | ERO cell line | The fabricated scaffolds displayed a greater capacity for whole blood clotting. | [134] |
| Hyaluronan Nanofiber | 25 | E. coli and S. aureus | Cell line (NIH 3T3) | Smaller particles have a greater impact on microorganisms, as evidenced by the nanoparticles size. | [135] |
| Activated Carbon coated silver nanocomposite | 50–400 | S. aureus, Klebsiella pneumoniae and P. aeruginosa | - | The Ag composites showed an increase in antibacterial activity when compared to the neat, activated carbon. | [136] |
| Polyurethane Foam mixed Ag-NPs Dressing | 100 | E. coli, P. aeruginosa and S. aureus | Human fibroblast | The foam dressing showed improved wound healing | [137] |
7. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lazarus, G.S.; Cooper, D.M.; Knighton, D.R.; Percoraro, R.E.; Rodeheaver, G.; Robson, M.C. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen. 1994, 2, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.C.; Steed, D.L.; Franz, M.G. Wound healing: Biologic features and approaches to maximize healing trajectories. Curr. Probl. Surg. 2001, 38, 72–140. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.; Holden, J.; Grob, M.; Soldin, M. Management of wounds in the community: Five principles. Br. J. Community Nurs. 2019, 24, S20–S23. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health J. Int. Soc. Pharm. Outcomes Res. 2018, 21, 27–32. [Google Scholar] [CrossRef]
- AWCS. Wound Care Research and the Imperative for Funding. Available online: https://www.woundcarestakeholders.org/value-of-wound-care/research/wound-care-research-the-imperative-for-funding2021 (accessed on 15 March 2022).
- Jakucs, C. Wound Healing Research: The Need for Grants Is Widespread. Available online: https://blog.wcei.net/2020/10/wound-healing-research-the-need-for-grantsis-widespread (accessed on 5 March 2022).
- Armstrong, D.G.; Swerdlow, M.A.; Armstrong, A.A.; Conte, M.S.; Padula, W.V.; Bus, S.A. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J. Foot Ankle Res. 2020, 13, 16. [Google Scholar] [CrossRef]
- Wound Source—Venous Leg Ulcer Chronicity and Recurrence: Breaking the Cycle. Available online: https://www.woundsource.com/blog/venous-leg-ulcer-chroniccity-and-recurrence-breaking-cycle (accessed on 8 March 2022).
- Venous Leg Ulcer (VLU) Treatment Market. Available online: https://www.fortunebusinessinsights.com/venous-leg-ulcervlu-treatment-market (accessed on 25 February 2022).
- Attinger, C.E.; Janis, J.E.; Steinberg, J.; Schwartz, J.; Al-Attar, A.; Couch, K. Clinical approach to wounds: Debridement and wound bed preparation including the use of dressings and wound-healing adjuvants. Plast. Reconstr. Surg. 2006, 117, 72S–109S. [Google Scholar] [CrossRef]
- Degreef, H.J. How to heal a wound fast. Dermatol. Clin. 1998, 16, 365–375. [Google Scholar] [CrossRef]
- MacNeil, S. Progress and opportunities for tissue-engineered skin. Nature 2007, 445, 874–880. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Chouhan, D.; Mandal, B.B. Tissue Engineered Skin and Wound Healing: Current Strategies and Future Directions. Curr. Pharm. Des. 2017, 23, 3455–3482. [Google Scholar] [CrossRef]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflam. 2019, 2019, 3706315. [Google Scholar] [CrossRef]
- Kapoor, M.; Appleton, I. Wound healing: Abnormalities and future therapeutic targets. Curr. Anaesth. Crit. Care 2005, 16, 88–93. [Google Scholar] [CrossRef]
- Moura, L.I.; Dias, A.M.; Carvalho, E.; de Sousa, H.C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—A review. Acta Biomater. 2013, 9, 7093–7114. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Morton, L.M.; Phillips, T.J. Wound healing and treating wounds: Differential diagnosis and evaluation of chronic wounds. J. Am. Acad. Dermatol. 2016, 74, 589–605; quiz 605–586. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef]
- Lin, Y.H.; Hsu, W.S.; Chung, W.Y.; Ko, T.H.; Lin, J.H. Silver-based wound dressings reduce bacterial burden and promote wound healing. Int Wound J. 2016, 13, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Leaper, D.; Assadian, O.; Edmiston, C.E. Approach to chronic wound infections. Br. J. Dermatol. 2015, 173, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Bessa, L.J.; Fazii, P.; Di Giulio, M.; Cellini, L. Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: Some remarks about wound infection. Int Wound J. 2015, 12, 47–52. [Google Scholar] [CrossRef]
- Bassetti, M.; Vena, A.; Croxatto, A.; Righi, E.; Guery, B. How to manage Pseudomonas aeruginosa infections. Drugs Context 2018, 7, 212527. [Google Scholar] [CrossRef]
- Khan, H.A.; Ahmad, A.; Mehboob, R. Nosocomial infections and their control strategies. Asian Pac. J. Trop. Biomed. 2015, 5, 509–514. [Google Scholar] [CrossRef] [Green Version]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Werth, B.J.; Sakoulas, G.; Rose, W.E.; Pogliano, J.; Tewhey, R.; Rybak, M.J. Ceftaroline increases membrane binding and enhances the activity of daptomycin against daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus in a pharmacokinetic/pharmacodynamic model. Antimicrob. Agents Chemother. 2013, 57, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Steed, M.E.; Vidaillac, C.; Rybak, M.J. Novel daptomycin combinations against daptomycin-nonsusceptible methicillin-resistant Staphylococcus aureus in an in vitro model of simulated endocardial vegetations. Antimicrob. Agents Chemother. 2010, 54, 5187–5192. [Google Scholar] [CrossRef]
- Rose, W.E.; Berti, A.D.; Hatch, J.B.; Maki, D.G. Relationship of in vitro synergy and treatment outcome with daptomycin plus rifampin in patients with invasive methicillin-resistant Staphylococcus aureus infections. Antimicrob. Agents Chemother. 2013, 57, 3450–3452. [Google Scholar] [CrossRef]
- Miro, J.M.; Entenza, J.M.; Del Rio, A.; Velasco, M.; Castaneda, X.; Garcia de la Maria, C.; Giddey, M.; Armero, Y.; Pericas, J.M.; Cervera, C.; et al. High-dose daptomycin plus fosfomycin is safe and effective in treating methicillin-susceptible and methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob. Agents Chemother. 2012, 56, 4511–4515. [Google Scholar] [CrossRef]
- Boucher, H.W.; Wilcox, M.; Talbot, G.H.; Puttagunta, S.; Das, A.F.; Dunne, M.W. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N. Engl. J. Med. 2014, 370, 2169–2179. [Google Scholar] [CrossRef]
- Moran, G.J.; Fang, E.; Corey, G.R.; Das, A.F.; De Anda, C.; Prokocimer, P. Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): A randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 2014, 14, 696–705. [Google Scholar] [CrossRef]
- Corey, G.R.; Kabler, H.; Mehra, P.; Gupta, S.; Overcash, J.S.; Porwal, A.; Giordano, P.; Lucasti, C.; Perez, A.; Good, S.; et al. Single-dose oritavancin in the treatment of acute bacterial skin infections. N. Engl. J. Med. 2014, 370, 2180–2190. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C.; et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, e10–e52. [Google Scholar] [CrossRef]
- Petkovsek, Z.; Elersic, K.; Gubina, M.; Zgur-Bertok, D.; Starcic Erjavec, M. Virulence potential of Escherichia coli isolates from skin and soft tissue infections. J. Clin. Microbiol. 2009, 47, 1811–1817. [Google Scholar]
- Zhou, Y.F.; Liu, P.; Zhang, C.J.; Liao, X.P.; Sun, J.; Liu, Y.H. Colistin Combined with Tigecycline: A Promising Alternative Strategy to Combat Escherichia coli Harboring bla NDM- 5 and mcr-1. Front. Microbiol. 2019, 10, 2957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjum, S.; Arora, A.; Alam, M.S.; Gupta, B. Development of antimicrobial and scar preventive chitosan hydrogel wound dressings. Int. J. Pharm. 2016, 508, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Michalska-Sionkowska, M.; Kaczmarek, B.; Walczak, M.; Sionkowska, A. Antimicrobial activity of new materials based on the blends of collagen/chitosan/hyaluronic acid with gentamicin sulfate addition. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 86, 103–108. [Google Scholar] [CrossRef]
- Radulescu, M.; Holban, A.M.; Mogoanta, L.; Balseanu, T.A.; Mogosanu, G.D.; Savu, D.; Popescu, R.C.; Fufa, O.; Grumezescu, A.M.; Bezirtzoglou, E.; et al. Fabrication, Characterization, and Evaluation of Bionanocomposites Based on Natural Polymers and Antibiotics for Wound Healing Applications. Molecules 2016, 21, 761. [Google Scholar] [CrossRef]
- Ahmadi, M.; Adibhesami, M. The Effect of Silver Nanoparticles on Wounds Contaminated with Pseudomonas aeruginosa in Mice: An Experimental Study. Iran. J. Pharm. Res. 2017, 16, 661–669. [Google Scholar]
- Anjum, A.; Sim, C.H.; Ng, S.F. Hydrogels Containing Antibiofilm and Antimicrobial Agents Beneficial for Biofilm-Associated Wound Infection: Formulation Characterizations and In vitro Study. AAPS PharmSciTech 2018, 19, 1219–1230. [Google Scholar] [CrossRef]
- Ahire, J.J.; Robertson, D.D.; van Reenen, A.J.; Dicks, L.M. Polyethylene oxide (PEO)-hyaluronic acid (HA) nanofibers with kanamycin inhibits the growth of Listeria monocytogenes. Biomed. Pharm. 2017, 86, 143–148. [Google Scholar] [CrossRef]
- Chapelle, C.; Gaborit, B.; Dumont, R.; Dinh, A.; Vallee, M. Treatment of UTIs Due to Klebsiella pneumoniae Carbapenemase-Producers: How to Use New Antibiotic Drugs? A Narrative Review. Antibiotics 2021, 10, 1332. [Google Scholar] [CrossRef]
- Webber, B.J.; Kieffer, J.W.; White, B.K.; Hawksworth, A.W.; Graf, P.C.F.; Yun, H.C. Chemoprophylaxis against group A Streptococcus during military training. Prev. Med. 2019, 118, 142–149. [Google Scholar] [CrossRef]
- Wasfi, R.; Hamed, S.M.; Amer, M.A.; Fahmy, L.I. Proteus mirabilis Biofilm: Development and Therapeutic Strategies. Front. Cell Infect. Microbiol. 2020, 10, 414. [Google Scholar] [CrossRef]
- Beganovic, M.; Luther, M.K.; Rice, L.B.; Arias, C.A.; Rybak, M.J.; LaPlante, K.L. A Review of Combination Antimicrobial Therapy for Enterococcus faecalis Bloodstream Infections and Infective Endocarditis. Clin. Infect. Dis. 2018, 67, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Lakticova, V.; Hutton-Thomas, R.; Meyer, M.; Gurkan, E.; Rice, L.B. Antibiotic-induced enterococcal expansion in the mouse intestine occurs throughout the small bowel and correlates poorly with suppression of competing flora. Antimicrob. Agents Chemother. 2006, 50, 3117–3123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panagiotidis, G.; Backstrom, T.; Asker-Hagelberg, C.; Jandourek, A.; Weintraub, A.; Nord, C.E. Effect of ceftaroline on normal human intestinal microflora. Antimicrob. Agents Chemother. 2010, 54, 1811–1814. [Google Scholar] [CrossRef]
- Smith, J.R.; Barber, K.E.; Raut, A.; Aboutaleb, M.; Sakoulas, G.; Rybak, M.J. beta-Lactam combinations with daptomycin provide synergy against vancomycin-resistant Enterococcus faecalis and Enterococcus faecium. J. Antimicrob. Chemother. 2015, 70, 1738–1743. [Google Scholar] [CrossRef]
- Chard, R. Wound classifications. AORN J. 2008, 88, 108. [Google Scholar]
- Bielefeld, K.A.; Amini-Nik, S.; Alman, B.A. Cutaneous wound healing: Recruiting developmental pathways for regeneration. Cell. Mol. Life Sci. 2013, 70, 2059–2081. [Google Scholar] [CrossRef]
- Simpson, D.M.; Ross, R. The neutrophilic leukocyte in wound repair a study with antineutrophil serum. J. Clin. Invest. 1972, 51, 2009–2023. [Google Scholar] [CrossRef]
- Albelda, S.M.; Buck, C.A. Integrins and other cell adhesion molecules. FASEB J. 1990, 4, 2868–2880. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Schaffer, M.; Barbul, A. Lymphocyte function in wound healing and following injury. Br. J. Surg 1998, 85, 444–460. [Google Scholar] [CrossRef]
- Braund, R.; Hook, S.; Medlicott, N.J. The role of topical growth factors in chronic wounds. Curr. Drug Deliv. 2007, 4, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Gainza, G.; Villullas, S.; Pedraz, J.L.; Hernandez, R.M.; Igartua, M. Advances in drug delivery systems (DDSs) to release growth factors for wound healing and skin regeneration. Nanomedicine 2015, 11, 1551–1573. [Google Scholar] [CrossRef]
- Kiwanuka, E.; Junker, J.; Eriksson, E. Harnessing growth factors to influence wound healing. Clin. Plast. Surg. 2012, 39, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Hunt, T.K.; Hopf, H.; Hussain, Z. Physiology of wound healing. Adv. Ski. Wound Care 2000, 13, 6–11. [Google Scholar] [CrossRef]
- Martins, V.L.; Caley, M.; O’Toole, E.A. Matrix metalloproteinases and epidermal wound repair. Cell Tissue Res. 2013, 351, 255–268. [Google Scholar] [CrossRef]
- Mihai, M.M.; Holban, A.M.; Giurcaneanu, C.; Popa, L.G.; Buzea, M.; Filipov, M.; Lazar, V.; Chifiriuc, M.C.; Popa, M.I. Identification and phenotypic characterization of the most frequent bacterial etiologies in chronic skin ulcers. Rom. J. Morphol. Embryol. 2014, 55, 1401–1408. [Google Scholar]
- Saurav, K.; Bar-Shalom, R.; Haber, M.; Burgsdorf, I.; Oliviero, G.; Costantino, V.; Morgenstern, D.; Steindler, L. In Search of Alternative Antibiotic Drugs: Quorum-Quenching Activity in Sponges and their Bacterial Isolates. Front. Microbiol. 2016, 7, 416. [Google Scholar] [CrossRef]
- Sakarikou, C.; Kostoglou, D.; Simões, M.; Giaouris, E. Exploitation of plant extracts and phytochemicals against resistant Salmonella spp. in biofilms. Food Res. Int. 2020, 128, 108806. [Google Scholar] [CrossRef]
- Haroun, M.F.; Al-Kayali, R.S. Synergistic effect of Thymbra spicata L. extracts with antibiotics against multidrug- resistant Staphylococcus aureus and Klebsiella pneumoniae strains. Iran. J. Basic Med. Sci. 2016, 19, 1193–1200. [Google Scholar]
- Revathy, T.; Saranya, R.; Jayasri, M.A.; Saurav, K.; Suthindhiran, K. Morphological alterations in erythrocytes treated with silver nanoparticles biomineralized by marine sediment-derived Bacillus sp. VITSSN01. Ann. Microbiol. 2014, 64, 1291–1299. [Google Scholar] [CrossRef]
- Lansdown, A.B. Physiological and toxicological changes in the skin resulting from the action and interaction of metal ions. Crit. Rev. Toxicol. 1995, 25, 397–462. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.B. A pharmacological and toxicological profile of silver as an antimicrobial agent in medical devices. Adv. Pharmacol. Sci. 2010, 2010, 910686. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhu, H.; Wang, X.; Cui, D.; Gao, Z.; Su, D.; Zhao, J.; Chen, O. Cu-Catalyzed Synthesis of CdZnSe-CdZnS Alloy Quantum Dots with Highly Tunable Emission. Chem. Mater. 2019, 31, 2635–2643. [Google Scholar] [CrossRef]
- Khorrami, S.; Zarrabi, A.; Khaleghi, M.; Danaei, M.; Mozafari, M.R. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomed. 2018, 13, 8013–8024. [Google Scholar] [CrossRef] [Green Version]
- Ramkumar, V.S.; Pugazhendhi, A.; Gopalakrishnan, K.; Sivagurunathan, P.; Saratale, G.D.; Dung, T.N.B.; Kannapiran, E. Biofabrication and characterization of silver nanoparticles using aqueous extract of seaweed Enteromorpha compressa and its biomedical properties. Biotechnol. Rep. 2017, 14, 1–7. [Google Scholar] [CrossRef]
- Durán, N.; Nakazato, G.; Seabra, A.B. Antimicrobial activity of biogenic silver nanoparticles, and silver chloride nanoparticles: An overview and comments. Appl. Microbiol. Biotechnol. 2016, 100, 6555–6570. [Google Scholar] [CrossRef]
- Shankar, P.D.; Shobana, S.; Karuppusamy, I.; Pugazhendhi, A.; Ramkumar, V.S.; Arvindnarayan, S.; Kumar, G. A review on the biosynthesis of metallic nanoparticles (gold and silver) using bio-components of microalgae: Formation mechanism and applications. Enzym. Microb Technol. 2016, 95, 28–44. [Google Scholar] [CrossRef]
- Rozhin, A.; Batasheva, S.; Kruychkova, M.; Cherednichenko, Y.; Rozhina, E.; Fakhrullin, R. Biogenic Silver Nanoparticles: Synthesis and Application as Antibacterial and Antifungal Agents. Micromachines 2021, 12, 1480. [Google Scholar] [CrossRef]
- Naskar, A.; Kim, K.S. Recent Advances in Nanomaterial-Based Wound-Healing Therapeutics. Pharmaceutics 2020, 12, 499. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Metal Oxide Nanoparticles as Biomedical Materials. Biomimetics 2020, 5, 27. [Google Scholar] [CrossRef]
- Ferro, C.; Florindo, H.F.; Santos, H.A. Selenium Nanoparticles for Biomedical Applications: From Development and Characterization to Therapeutics. Adv. Healthc. Mater. 2021, 10, 2100598. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Sim, S.; Wong, N.K. Nanotechnology and its use in imaging and drug delivery (Review). Biomed. Rep. 2021, 14, 42. [Google Scholar] [CrossRef]
- Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on Nanoparticles and Nanostructured Materials: Bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications. Nanomaterials 2022, 12, 457. [Google Scholar] [CrossRef]
- Gaillet, S.; Rouanet, J.M. Silver nanoparticles: Their potential toxic effects after oral exposure and underlying mechanisms—A review. Food Chem. Toxicol. 2015, 77, 58–63. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Kędziora, A.; Wieczorek, R.; Speruda, M.; Matolínová, I.; Goszczyński, T.M.; Litwin, I.; Matolín, V.; Bugla-Płoskońska, G. Comparison of Antibacterial Mode of Action of Silver Ions and Silver Nanoformulations with Different Physico-Chemical Properties: Experimental and Computational Studies. Front. Microbiol. 2021, 12, 659614. [Google Scholar] [CrossRef]
- Raczkowska, J.; Stetsyshyn, Y.; Awsiuk, K.; Brzychczy-Włoch, M.; Gosiewski, T.; Jany, B.; Lishchynskyi, O.; Shymborska, Y.; Nastyshyn, S.; Bernasik, A.; et al. “Command” surfaces with thermo-switchable antibacterial activity. Mater. Sci. Eng. C 2019, 103, 109806. [Google Scholar] [CrossRef]
- Hemeg, H.A. Nanomaterials for alternative antibacterial therapy. Int. J. Nanomed. 2017, 12, 8211–8225. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- Konnova, S.A.; Danilushkina, A.A.; Fakhrullina, G.I.; Akhatova, F.S.; Badrutdinov, A.R.; Fakhrullin, R.F. Silver nanoparticle-coated “cyborg” microorganisms: Rapid assembly of polymer-stabilised nanoparticles on microbial cells. RSC Adv. 2015, 5, 13530–13537. [Google Scholar] [CrossRef]
- Tarhan, T.; Şen, Ö.; Ciofani, M.E.; Yılmaz, D.; Çulha, M. Synthesis and characterization of silver nanoparticles decorated polydopamine coated hexagonal boron nitride and its effect on wound healing. J. Trace Elem. Med. Biol. 2021, 67, 126774. [Google Scholar] [CrossRef]
- Liu, X.; Lee, P.Y.; Ho, C.M.; Lui, V.C.; Chen, Y.; Che, C.M.; Tam, P.K.; Wong, K.K. Silver nanoparticles mediate differential responses in keratinocytes and fibroblasts during skin wound healing. ChemMedChem 2010, 5, 468–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shevtsova, T.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Donchak, V.; Harhay, K.; Korolko, S.; Budkowski, A.; Stetsyshyn, Y. Temperature-responsive hybrid nanomaterials based on modified halloysite nanotubes uploaded with silver nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128525. [Google Scholar] [CrossRef]
- Thanh, N.T.; Hieu, M.H.; Phuong, N.T.M.; Thuan, T.D.B.; Thu, H.N.T.; Thai, V.-P.; Minh, T.D.; Dai, H.N.; Vo, V.T.; Thi, H.N. Optimization and characterization of electrospun polycaprolactone coated with gelatin-silver nanoparticles for wound healing application. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 318–329. [Google Scholar] [CrossRef]
- Fong, J.; Wood, F. Nanocrystalline silver dressings in wound management: A review. Int J. Nanomed. 2006, 1, 441–449. [Google Scholar] [CrossRef]
- Hamdan, S.; Pastar, I.; Drakulich, S.; Dikici, E.; Tomic-Canic, M.; Deo, S.; Daunert, S. Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Cent. Sci. 2017, 3, 163–175. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int. J. Biol. Macromol. 2019, 122, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Szmyd, R.; Goralczyk, A.G.; Skalniak, L.; Cierniak, A.; Lipert, B.; Filon, F.L.; Crosera, M.; Borowczyk, J.; Laczna, E.; Drukala, J.; et al. Effect of silver nanoparticles on human primary keratinocytes. Biol. Chem. 2013, 394, 113–123. [Google Scholar] [CrossRef]
- Pal, S.; Nisi, R.; Stoppa, M.; Licciulli, A. Silver-Functionalized Bacterial Cellulose as Antibacterial Membrane for Wound-Healing Applications. ACS Omega 2017, 2, 3632–3639. [Google Scholar] [CrossRef] [PubMed]
- Hanif, Z.; Khan, Z.A.; Siddiqui, M.F.; Tariq, M.Z.; Park, S.; Park, S.J. Tannic acid-mediated rapid layer-by-layer deposited non-leaching silver nanoparticles hybridized cellulose membranes for point-of-use water disinfection. Carbohydr. Polym. 2020, 231, 115746. [Google Scholar] [CrossRef]
- Dong, X.; Shannon, H.D.; Amirsoleimani, A.; Brion, G.M.; Escobar, I.C. Thiol-Affinity Immobilization of Casein-Coated Silver Nanoparticles on Polymeric Membranes for Biofouling Control. Polymers 2019, 11, 2057. [Google Scholar] [CrossRef]
- Levi-Polyachenko, N.; Jacob, R.; Day, C.; Kuthirummal, N. Chitosan wound dressing with hexagonal silver nanoparticles for hyperthermia and enhanced delivery of small molecules. Colloids Surf. B Biointerfaces 2016, 142, 315–324. [Google Scholar] [CrossRef]
- Singh, R.; Singh, D. Chitin membranes containing silver nanoparticles for wound dressing application. Int. Wound J. 2014, 11, 264–268. [Google Scholar] [CrossRef]
- Chen, X.; Lin, H.; Xu, T.; Lai, K.; Han, X.; Lin, M. Cellulose nanofibers coated with silver nanoparticles as a flexible nanocomposite for measurement of flusilazole residues in Oolong tea by surface-enhanced Raman spectroscopy. Food Chem. 2020, 315, 126276. [Google Scholar] [CrossRef]
- Yun, B.J.; Koh, W.-G. Highly-sensitive SERS-based immunoassay platform prepared on silver nanoparticle-decorated electrospun polymeric fibers. J. Ind. Eng. Chem. 2020, 82, 341–348. [Google Scholar] [CrossRef]
- Shen, B.; Zhang, D.; Wei, Y.; Zhao, Z.; Ma, X.; Zhao, X.; Wang, S.; Yang, W. Preparation of Ag Doped Keratin/PA6 Nanofiber Membrane with Enhanced Air Filtration and Antimicrobial Properties. Polymers 2019, 11, 1511. [Google Scholar] [CrossRef]
- Dou, J.; Zhu, G.; Hu, B.; Yang, J.; Ge, Y.; Li, X.; Liu, J. Wall thickness-tunable AgNPs-NCNTs for hydrogen peroxide sensing and oxygen reduction reaction. Electrochim. Acta 2019, 306, 466–476. [Google Scholar]
- Liu, J.; Sonshine, D.A.; Shervani, S.; Hurt, R.H. Controlled release of biologically active silver from nanosilver surfaces. ACS Nano 2010, 4, 6903–6913. [Google Scholar] [PubMed]
- Tian, J.; Wong, K.K.; Ho, C.M.; Lok, C.N.; Yu, W.Y.; Che, C.M.; Chiu, J.F.; Tam, P.K. Topical delivery of silver nanoparticles promotes wound healing. ChemMedChem 2007, 2, 129–136. [Google Scholar] [PubMed]
- Yeh, Y.C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar]
- Lu, M.M.; Bai, J.; Shao, D.; Qiu, J.; Li, M.; Zheng, X.; Xiao, Y.; Wang, Z.; Chang, Z.M.; Chen, L.; et al. Antibacterial and biodegradable tissue nano-adhesives for rapid wound closure. Int. J. Nanomed. 2018, 13, 5849–5863. [Google Scholar]
- El-Aassar, M.R.; Ibrahim, O.M.; Fouda, M.M.G.; El-Beheri, N.G.; Agwa, M.M. Wound healing of nanofiber comprising Polygalacturonic/Hyaluronic acid embedded silver nanoparticles: In-vitro and in-vivo studies. Carbohydr. Polym. 2020, 238, 116175. [Google Scholar]
- Warren, D.S.; Sutherland, S.P.H.; Kao, J.Y.; Weal, G.R.; Mackay, S.M. The Preparation and Simple Analysis of a Clay Nanoparticle Composite Hydrogel. J. Chem. Educ. 2017, 94, 1772–1779. [Google Scholar]
- Haleem, A.; Chen, J.; Guo, X.-X.; Wang, J.-Y.; Li, H.-J.; Li, P.-Y.; Chen, S.-Q.; He, W.-D. Hybrid cryogels composed of P(NIPAM-co-AMPS) and metal nanoparticles for rapid reduction of p-nitrophenol. Polymer 2020, 193, 122352. [Google Scholar]
- Atefeh, S. Design of AgNPs -Base Starch/PEG-Poly (Acrylic Acid) Hydrogel for Removal of Mercury (II). J. Polym. Environ. 2020, 28, 906–917. [Google Scholar]
- Dil, N.N.; Sadeghi, M. Free radical synthesis of nanosilver/gelatin-poly (acrylic acid) nanocomposite hydrogels employed for antibacterial activity and removal of Cu(II) metal ions. J. Hazard. Mater. 2018, 351, 38–53. [Google Scholar]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar]
- Rahmani, S.; Mooney, D. Tissue-Engineered Wound Dressings for Diabetic Foot Ulcers: Medical and Surgical Management. In The Diabetic Foot. Contemporary Diabetes; Veves, A., Giurini, J., Guzman, R., Eds.; Humana: Cham, Switzerland, 2018; Volume 15, pp. 247–256. [Google Scholar]
- Tate, S.; Price, A.; Harding, K. Dressings for venous leg ulcers. BMJ 2018, 361, k1604. [Google Scholar]
- Weller, C. 4—Interactive dressings and their role in moist wound management. In Advanced Textiles for Wound Care; Rajendran, S., Ed.; Woodhead Publishing: Sawston, UK, 2009; pp. 97–113. [Google Scholar]
- Weller, C.D.; Team, V.; Sussman, G. First-Line Interactive Wound Dressing Update: A Comprehensive Review of the Evidence. Front. Pharm. 2020, 11, 155. [Google Scholar]
- Hubner, P.; Donati, N.; Quines, L.K.d.M.; Tessaro, I.C.; Marcilio, N.R. Gelatin-based films containing clinoptilolite-Ag for application as wound dressing. Mater. Sci. Eng. C 2020, 107, 110215. [Google Scholar]
- Ambrogi, V.; Pietrella, D.; Donnadio, A.; Latterini, L.; Di Michele, A.; Luffarelli, I.; Ricci, M. Biocompatible alginate silica supported silver nanoparticles composite films for wound dressing with antibiofilm activity. Mater. Sci. Eng. C 2020, 112, 110863. [Google Scholar]
- An, J.; Zhang, H.; Zhang, J.; Zhao, Y.; Yuan, X. Preparation and antibacterial activity of electrospun chitosan/poly(ethylene oxide) membranes containing silver nanoparticles. Colloid Polym. Sci. 2009, 287, 1425–1434. [Google Scholar]
- Archana, D.; Singh, B.K.; Dutta, J.; Dutta, P.K. Chitosan-PVP-nano silver oxide wound dressing: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2015, 73, 49–57. [Google Scholar]
- Montaser, A.S.; Abdel-Mohsen, A.M.; Ramadan, M.A.; Sleem, A.A.; Sahffie, N.M.; Jancar, J.; Hebeish, A. Preparation and characterization of alginate/silver/nicotinamide nanocomposites for treating diabetic wounds. Int. J. Biol. Macromol. 2016, 92, 739–747. [Google Scholar]
- Lu, B.; Lu, F.; Zou, Y.; Liu, J.; Rong, B.; Li, Z.; Dai, F.; Wu, D.; Lan, G. In situ reduction of silver nanoparticles by chitosan-l-glutamic acid/hyaluronic acid: Enhancing antimicrobial and wound-healing activity. Carbohydr. Polym. 2017, 173, 556–565. [Google Scholar]
- Ye, D.; Zhong, Z.; Xu, H.; Chang, C.; Yang, Z.; Wang, Y.; Ye, Q.; Zhang, L. Construction of cellulose/nanosilver sponge materials and their antibacterial activities for infected wounds healing. Cellulose 2016, 23, 749–763. [Google Scholar]
- Shao, J.; Wang, B.; Li, J.; Jansen, J.A.; Walboomers, X.F.; Yang, F. Antibacterial effect and wound healing ability of silver nanoparticles incorporation into chitosan-based nanofibrous membranes. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zheng, Y.; Wen, X.; Lin, Q.; Chen, X.; Wu, Z. Silver nanoparticle/bacterial cellulose gel membranes for antibacterial wound dressing: Investigation in vitro and in vivo. Biomed. Mater. 2014, 9, 035005. [Google Scholar] [CrossRef] [PubMed]
- Verma, J.; Kanoujia, J.; Parashar, P.; Tripathi, C.B.; Saraf, S.A. Wound healing applications of sericin/chitosan-capped silver nanoparticles incorporated hydrogel. Drug Deliv. Transl. Res. 2017, 7, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Shan, X.; Zhao, X.; Zha, H.; Chen, X.; Wang, J.; Cai, C.; Wang, X.; Li, G.; Hao, J.; et al. Spongy bilayer dressing composed of chitosan-Ag nanoparticles and chitosan-Bletilla striata polysaccharide for wound healing applications. Carbohydr. Polym. 2017, 157, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Lu, Z.; Yang, H.; Gao, J.; Chen, R. Novel Asymmetric Wettable AgNPs/Chitosan Wound Dressing: In Vitro and In Vivo Evaluation. ACS Appl. Mater. Interfaces 2016, 8, 3958–3968. [Google Scholar] [CrossRef]
- Khampieng, T.; Brikshavana, P.; Supaphol, P. Silver nanoparticle-embedded poly(vinyl pyrrolidone) hydrogel dressing: Gamma-ray synthesis and biological evaluation. J. Biomater. Sci. Polym. Ed. 2014, 25, 826–842. [Google Scholar] [CrossRef]
- Perez-Diaz, M.; Alvarado-Gomez, E.; Magana-Aquino, M.; Sanchez-Sanchez, R.; Velasquillo, C.; Gonzalez, C.; Ganem-Rondero, A.; Martinez-Castanon, G.; Zavala-Alonso, N.; Martinez-Gutierrez, F. Anti-biofilm activity of chitosan gels formulated with silver nanoparticles and their cytotoxic effect on human fibroblasts. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 317–323. [Google Scholar]
- Kumar, P.T.S.; Abhilash, S.; Manzoor, K.; Nair, S.V.; Tamura, H.; Jayakumar, R. Preparation and characterization of novel β-chitin/nanosilver composite scaffolds for wound dressing applications. Carbohydr. Polym. 2010, 80, 761–767. [Google Scholar] [CrossRef]
- Abdel-Mohsen, A.M.; Hrdina, R.; Burgert, L.; Abdel-Rahman, R.M.; Hasova, M.; Smejkalova, D.; Kolar, M.; Pekar, M.; Aly, A.S. Antibacterial activity and cell viability of hyaluronan fiber with silver nanoparticles. Carbohydr. Polym. 2013, 92, 1177–1187. [Google Scholar] [CrossRef]
- Kim, M.H.; Cho, D.; Kwon, O.H.; Park, W.H. Thermal fabrication and characterization of Ag nanoparticle-activated carbon composites for functional wound-dressing additives. J. Alloys Compd. 2018, 735, 2670–2674. [Google Scholar] [CrossRef]
- Namviriyachote, N.; Lipipun, V.; Akkhawattanangkul, Y.; Charoonrut, P.; Ritthidej, G.C. Development of polyurethane foam dressing containing silver and asiaticoside for healing of dermal wound. Asian J. Pharm. Sci. 2019, 14, 63–77. [Google Scholar] [CrossRef]



| Causative Bacteria | Wound Types | Diseases/Infections Associated | Preventive Measures | Drugs | References |
|---|---|---|---|---|---|
| Staphylococcus aureus | Acute | Abscesses (boils), Furuncles, Cellulitis | Maintaining good hygiene and regular and frequent hand washing | β-lactam antibiotics, Vancomycin, Daptomycin, Linezolid, Rifampin, and Tedizolid. | [25,26,27,28,29,30,31,32,33] |
| Escherichia coli | Clinical | Surgical site infections, Neonatal omphalitis and necrotizing fasciitis | Wash hands before handling, serving, or eating food, and especially after touching animals, working with livestock | Ciprofloxacin, Amoxicillin, Colistin, Tetracycline, Gentamicin and Cefuroxime | [19,34,35,36,37,38] |
| Pseudomonas aeruginosa | Open | Chronic wounds, pneumonia and UTIs | 1% acetic acid is a simple, safe, and effective topical antiseptic that can be used in the elimination of P. aeruginosa from chronic infected wounds | Ciprofloxacin, Gentamicin and Kanamycin | [19,39,40,41] |
| Klebsiellia pneumonia | Chronic | UTIs | Strict adherence to hand hygiene, wearing gowns and gloves | Meropenem and Vaborbactam | [42] |
| Streptococcus pyogens | Acute | Strep throat, pharyngitis, scarlet fever (rash), impetigo, cellulitis, or erysipelas. | Wash hands before handling, serving, or eating food | Penicillin | [43] |
| Proteus species | Surgical acute | UTIs | Minimizing the incidence of infection using urinary catheterization and using high spectrum antibiotics | Ciprofloxacin | [44] |
| Enterococcus faecalis | Surgical | Bacteremia, UTIs, catheter-related infections, pelvic infections. | Practicing good hygiene and using potent antibiotics | Ampicillin Cefepime, Ceftaroline and Daptomycin | [45,46,47,48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, M.; Thakur, V.; Kumar, V.; Raj, M.; Gupta, S.; Devi, N.; Upadhyay, S.K.; Macho, M.; Banerjee, A.; Ewe, D.; et al. Silver Nanoparticles and Its Mechanistic Insight for Chronic Wound Healing: Review on Recent Progress. Molecules 2022, 27, 5587. https://doi.org/10.3390/molecules27175587
Singh M, Thakur V, Kumar V, Raj M, Gupta S, Devi N, Upadhyay SK, Macho M, Banerjee A, Ewe D, et al. Silver Nanoparticles and Its Mechanistic Insight for Chronic Wound Healing: Review on Recent Progress. Molecules. 2022; 27(17):5587. https://doi.org/10.3390/molecules27175587
Chicago/Turabian StyleSingh, Manoj, Vanita Thakur, Vikas Kumar, Mayank Raj, Shivani Gupta, Nisha Devi, Sushil Kumar Upadhyay, Markéta Macho, Avik Banerjee, Daniela Ewe, and et al. 2022. "Silver Nanoparticles and Its Mechanistic Insight for Chronic Wound Healing: Review on Recent Progress" Molecules 27, no. 17: 5587. https://doi.org/10.3390/molecules27175587
APA StyleSingh, M., Thakur, V., Kumar, V., Raj, M., Gupta, S., Devi, N., Upadhyay, S. K., Macho, M., Banerjee, A., Ewe, D., & Saurav, K. (2022). Silver Nanoparticles and Its Mechanistic Insight for Chronic Wound Healing: Review on Recent Progress. Molecules, 27(17), 5587. https://doi.org/10.3390/molecules27175587