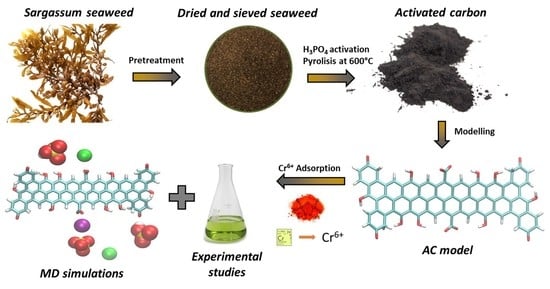
Adsorption of Hexavalent Chromium Using Activated Carbon Produced from Sargassum ssp.: Comparison between Lab Experiments and Molecular Dynamics Simulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Sargassum AC
2.3. Adsorbents Characterisation
2.4. Batch Adsorption Studies
2.5. Molecular Dynamics Simulations
3. Results and Discussion
3.1. Textural Characterization
Surface Area and Pore Size Distribution
3.2. Chemical Characterization
3.2.1. pHPZC
3.2.2. Boehm Analysis
3.2.3. FTIR Analysis
3.2.4. XPS Analysis
3.3. Adsorption Experiments
3.3.1. Influence of Different Parameters on Chromium Adsorption
3.3.2. Influence of Solution pH
3.3.3. Influence of Solution Temperature and Thermodynamic Studies
3.3.4. Influence of AC Pre-Treatment with Magnesium Chloride
3.3.5. Adsorption Kinetics
3.3.6. Adsorption Isotherms
3.4. Molecular Dynamics Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, M.; Hu, C.; Barnes, B.B.; Mitchum, G.; Lapointe, B.; Montoya, J.P. The great Atlantic Sargassum belt. Science 2019, 365, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Desrochers, A. Sargassum Uses Guide: A Resource for Caribbean Researchers, Entrepreneurs and Policy Makers; CERMES Technical Report; Cave Hill: Barbados, Caribbean, 2020. [Google Scholar]
- Liranzo-Gómez, R.E.; García-Cortés, D.; Jáuregui-Haza, U. Adaptation and Sustainable Management of Massive Influx of Sargassum in the Caribbean. Procedia Environ. Sci. Eng. Manag. 2021, 8, 543–553. [Google Scholar]
- Barquilha, C.; Cossich, E.; Tavares, C.; Silva, E. Biosorption of nickel(II) and copper(II) ions by Sargassum sp. in nature and alginate extraction products. Bioresour. Technol. Rep. 2018, 5, 43–50. [Google Scholar] [CrossRef]
- He, J.; Chen, J.P. A comprehensive review on biosorption of heavy metals by algal biomass: Materials, performances, chemistry, and modeling simulation tools. Bioresour. Technol. 2014, 160, 67–78. [Google Scholar] [CrossRef]
- Ranguin, R.; Delannoy, M.; Yacou, C.; Jean-Marius, C.; Feidt, C.; Rychen, G.; Gaspard, S. Biochar and activated carbons preparation from invasive algae Sargassum spp. for Chlordecone availability reduction in contaminated soils. J. Environ. Chem. Eng. 2021, 9, 105280. [Google Scholar] [CrossRef]
- Francoeur, M.; Ferino-Pérez, A.; Yacou, C.; Jean-Marius, C.; Emmanuel, E.; Chérémond, Y.; Jauregui-Haza, U.; Gaspard, S. Activated carbon synthetized from Sargassum (sp) for adsorption of caffeine: Understanding the adsorption mechanism using molecular modeling. J. Environ. Chem. Eng. 2020, 9, 104795. [Google Scholar] [CrossRef]
- Esmaeili, A.; Ghasemi, S.; Zamani, F. Investigation of Cr(VI) Adsorption by Dried Brown Algae Sargassum Sp. and Its Activated Carbon. Iran. J. Chem. Chem. Eng. (IJCCE) 2012, 31, 11–19. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Y.; Cao, N.; Sun, D.; Zhang, Z.; Wang, Y.; Wen, Y.; Yang, Y.; Lyu, T. Sustainable Chromium (VI) Removal from Contaminated Groundwater Using Nano-Magnetite-Modified Biochar via Rapid Microwave Synthesis. Molecules 2020, 26, 103. [Google Scholar] [CrossRef]
- Tripathi, S.M.; Chaurasia, S. Detection of Chromium in surface and groundwater and its bio-absorption using bio-wastes and vermiculite. Eng. Sci. Technol. Int. J. 2020, 23, 1153–1161. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Fang, Q.; Li, Z.; Lai, Y.; Yang, H. Prepared PANI@nano hollow carbon sphere adsorbents with lappaceum shell like structure for high efficiency removal of hexavalent chromium. Chemosphere 2020, 263, 128109. [Google Scholar] [CrossRef]
- Singh, S.; Anil, A.G.; Khasnabis, S.; Kumar, V.; Nath, B.; Adiga, V.; Naik, T.S.K.; Subramanian, S.; Kumar, V.; Singh, J.; et al. Sustainable removal of Cr(VI) using graphene oxide-zinc oxide nanohybrid: Adsorption kinetics, isotherms and thermodynamics. Environ. Res. 2021, 203, 111891. [Google Scholar] [CrossRef]
- Gómez-Aguilar, D.L.; Esteban-Muñoz, J.A.; Rodríguez-Miranda, J.P.; Baracaldo-Guzmán, D.; Salcedo-Parra, O.J. Desorption of Coffee Pulp Used as an Adsorbent Material for Cr(III and VI) Ions in Synthetic Wastewater: A Preliminary Study. Molecules 2022, 27, 2170. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J. Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption electrocoagulation, electrochemical reduction, electrodialysis, electrodeionization, photocatalysis and nanotechnology: A review. Environ. Chem. Lett. 2020, 18, 2055–2068. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nižetić, S.; Cheng, C.K.; Luque, R.; Thomas, S.; Banh, T.L.; Pham, V.V.; Nguyen, X.P. Heavy metal removal by biomass-derived carbon nanotubes as a greener environmental remediation: A comprehensive review. Chemosphere 2021, 287, 131959. [Google Scholar] [CrossRef]
- Fang, Y.; Yang, K.; Zhang, Y.; Peng, C.; Robledo-Cabrera, A.; López-Valdivieso, A. Highly surface activated carbon to remove Cr(VI) from aqueous solution with adsorbent recycling. Environ. Res. 2021, 197, 111151. [Google Scholar] [CrossRef]
- Tu, B.; Wen, R.; Wang, K.; Cheng, Y.; Deng, Y.; Cao, W.; Zhang, K.; Tao, H. Efficient removal of aqueous hexavalent chromium by activated carbon derived from Bermuda grass. J. Colloid Interface Sci. 2019, 560, 649–658. [Google Scholar] [CrossRef]
- Tran, Q.H.; Tam, L.T.; Van Quy, N.; Phan, V.N.; Van Tuan, H.; Huy, T.Q.; Dinh, N.X.; Le, A.-T. Enhanced adsorption efficiency of inorganic chromium (VI) ions by using carbon-encapsulated hematite nanocubes. J. Sci. Adv. Mater. Devices 2020, 5, 392–399. [Google Scholar] [CrossRef]
- Xu, S.; Yu, W.; Liu, S.; Xu, C.; Li, J.; Zhang, Y. Adsorption of Hexavalent Chromium Using Banana Pseudostem Biochar and Its Mechanism. Sustainability 2018, 10, 4250. [Google Scholar] [CrossRef]
- Guo, X.; Liu, A.; Lu, J.; Niu, X.; Jiang, M.; Ma, Y.; Liu, X.; Li, M. Adsorption Mechanism of Hexavalent Chromium on Biochar: Kinetic, Thermodynamic, and Characterization Studies. ACS Omega 2020, 5, 27323–27331. [Google Scholar] [CrossRef]
- Asimakopoulos, G.; Baikousi, M.; Kostas, V.; Papantoniou, M.; Bourlinos, A.B.; Zbořil, R.; Karakassides, M.A.; Salmas, C.E. Nanoporous Activated Carbon Derived via Pyrolysis Process of Spent Coffee: Structural Characterization. Investigation of Its Use for Hexavalent Chromium Removal. Appl. Sci. 2020, 10, 8812. [Google Scholar] [CrossRef]
- Valdivia, A.E.O.; Osorio, C.M.; Rodríguez, Y.M.V. Preparation of Activated Carbon from Coffee Waste as an Adsorbent for the Removal of Chromium (III) from Water. Optimization for an Experimental Box-Behnken Design. Chemistry 2020, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Enniya, I.; Rghioui, L.; Jourani, A. Adsorption of hexavalent chromium in aqueous solution on activated carbon prepared from apple peels. Sustain. Chem. Pharm. 2018, 7, 9–16. [Google Scholar] [CrossRef]
- Politi, D.; Sidiras, D. Modified Spruce Sawdust for Sorption of Hexavalent Chromium in Batch Systems and Fixed-Bed Columns. Molecules 2020, 25, 5156. [Google Scholar] [CrossRef] [PubMed]
- Ntuli, T.; Mongwe, T.; Sikeyi, L.; Mkhari, O.; Coville, N.; Nxumalo, E.; Maubane-Nkadimeng, M. Removal of hexavalent chromium via an adsorption coupled reduction mechanism using olive oil derived carbon nano-onions. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100477. [Google Scholar] [CrossRef]
- Prabu, D.; Kumar, P.S.; Rathi, B.S.; Sathish, S.; Anand, K.V.; Kumar, J.A.; Mohammed, O.B.; Silambarasan, P. Feasibility of magnetic nano adsorbent impregnated with activated carbon from animal bone waste: Application for the chromium (VI) removal. Environ. Res. 2021, 203, 111813. [Google Scholar] [CrossRef]
- Sarojini, G.; Babu, S.V.; Rajamohan, N.; Kumar, P.S.; Rajasimman, M. Surface modified polymer-magnetic-algae nanocomposite for the removal of chromium- equilibrium and mechanism studies. Environ. Res. 2021, 201, 111626. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L. Biosorption performance of date palm empty fruit bunch wastes for toxic hexavalent chromium removal. Environ. Res. 2020, 187, 109694. [Google Scholar] [CrossRef]
- Kumar, A.; Jena, H.M. Adsorption of Cr(VI) from aqueous solution by prepared high surface area activated carbon from Fox nutshell by chemical activation with H3PO4. J. Environ. Chem. Eng. 2017, 5, 2032–2041. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, S.; Liu, Q.; Chen, Z.; Gu, J.; Zhu, C.; Lu, T.; Zhang, D.; Ma, J. N-doped porous carbon with magnetic particles formed in situ for enhanced Cr(VI) removal. Water Res. 2013, 47, 4188–4197. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Wu, Z.; Deng, Q.; Tu, W.; Dai, G.; Zeng, Z.; Deng, S. Enhanced Cr(VI) removal by polyethylenimine- and phosphorus-codoped hierarchical porous carbons. J. Colloid Interface Sci. 2018, 523, 110–120. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, M.; Ma, L.; Ren, J.; Ma, P.; Li, B.; Wu, N.; Song, Z.; Huang, L. Nitrogen and sulfur codoped micro-mesoporous carbon sheets derived from natural biomass for synergistic removal of chromium(VI): Adsorption behavior and computing mechanism. Sci. Total Environ. 2020, 730, 138930. [Google Scholar] [CrossRef]
- Emamy, F.; Bumajdad, A.; Lukaszewicz, J. Adsorption of Hexavalent Chromium and Divalent Lead Ions on the Nitrogen-Enriched Chitosan-Based Activated Carbon. Nanomaterials 2021, 11, 1907. [Google Scholar] [CrossRef]
- Zhang, H.; Yue, X.; Li, F.; Xiao, R.; Zhang, Y.; Gu, D. Preparation of rice straw-derived biochar for efficient cadmium removal by modification of oxygen-containing functional groups. Sci. Total Environ. 2018, 631-632, 795–802. [Google Scholar] [CrossRef]
- Ma, W.; Xie, L.; Dai, L.; Sun, G.; Chen, J.; Su, F.; Cao, Y.; Lei, H.; Kong, Q.; Chen, C.-M. Influence of phosphorus doping on surface chemistry and capacitive behaviors of porous carbon electrode. Electrochim. Acta 2018, 266, 420–430. [Google Scholar] [CrossRef]
- Borthakur, P.; Aryafard, M.; Zara, Z.; David, Ř.; Minofar, B.; Das, M.R.; Vithanage, M. Computational and experimental assessment of pH and specific ions on the solute solvent interactions of clay-biochar composites towards tetracycline adsorption: Implications on wastewater treatment. J. Environ. Manag. 2021, 283, 111989. [Google Scholar] [CrossRef]
- Wei, F.; Zhang, H.-F.; He, X.-J.; Ma, H.; Dong, S.-A.; Xie, X.-Y. Synthesis of porous carbons from coal tar pitch for high-performance supercapacitors. New Carbon Mater. 2019, 34, 132–139. [Google Scholar] [CrossRef]
- Poghossian, A. Determination of the pHpzc of insulators surface from capacitance–voltage characteristics of MIS and EIS structures. Sens. Actuators B Chem. 1997, 44, 551–553. [Google Scholar] [CrossRef]
- Şahin, Y.; Öztürk, A. Biosorption of chromium(VI) ions from aqueous solution by the bacterium Bacillus thuringiensis. Process Biochem. 2005, 40, 1895–1901. [Google Scholar] [CrossRef]
- Park, J.E.; Shin, J.-H.; Oh, W.; Choi, S.-J.; Kim, J.; Kim, C.; Jeon, J. Removal of Hexavalent Chromium(VI) from Wastewater Using Chitosan-Coated Iron Oxide Nanocomposite Membranes. Toxics 2022, 10, 98. [Google Scholar] [CrossRef]
- Durimel, A.; Altenor, S.; Miranda-Quintana, R.; Du Mesnil, P.C.; Jauregui-Haza, U.; Gadiou, R.; Gaspard, S. pH dependence of chlordecone adsorption on activated carbons and role of adsorbent physico-chemical properties. Chem. Eng. J. 2013, 229, 239–249. [Google Scholar] [CrossRef]
- Khan, T.; Manan, T.S.B.A.; Isa, M.H.; Ghanim, A.A.J.; Beddu, S.; Jusoh, H.; Iqbal, M.S.; Ayele, G.T.; Jami, M.S. Modeling of Cu(II) Adsorption from an Aqueous Solution Using an Artificial Neural Network (ANN). Molecules 2020, 25, 3263. [Google Scholar] [CrossRef]
- Sych, N.; Trofymenko, S.; Poddubnaya, O.; Tsyba, M.; Sapsay, V.; Klymchuk, D.; Puziy, A. Porous structure and surface chemistry of phosphoric acid activated carbon from corncob. Appl. Surf. Sci. 2012, 261, 75–82. [Google Scholar] [CrossRef]
- Zhang, Z.; Flaherty, D.W. Modified potentiometric titration method to distinguish and quantify oxygenated functional groups on carbon materials by pKa and chemical reactivity. Carbon 2020, 166, 436–445. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A Linear Constraint Solver for Molecular Simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef]
- Lindahl, E.; Hess, B.; Van Der Spoel, D. GROMACS 3.0: A package for molecular simulation and trajectory analysis. J. Mol. Model. 2001, 7, 306–317. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Kumar, K.V.; Gadipelli, S.; Wood, B.; Ramisetty, K.A.; Stewart, A.A.; Howard, C.A.; Brett, D.J.L.; Rodriguez-Reinoso, F. Characterization of the adsorption site energies and heterogeneous surfaces of porous materials. J. Mater. Chem. A 2019, 7, 10104–10137. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Liu, Z.; Adewuyi, Y.G.; Shi, S.; Chen, H.; Li, Y.; Liu, D.; Liu, Y. Removal of gaseous Hg0 using novel seaweed biomass-based activated carbon. Chem. Eng. J. 2019, 366, 41–49. [Google Scholar] [CrossRef]
- Ding, S.; Liu, Y. Adsorption of CO2 from flue gas by novel seaweed-based KOH-activated porous biochars. Fuel 2019, 260, 116382. [Google Scholar] [CrossRef]
- Khalil, K.M.; Elhamdy, W.A.; Elsamahy, A.A. Biomass derived P−doped activated carbon as nanostructured mesoporous adsorbent for chromium(VI) pollutants with pronounced functional efficiency and recyclability. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128553. [Google Scholar] [CrossRef]
- Yang, Y.; Cannon, F.S. Biomass activated carbon derived from pine sawdust with steam bursting pretreatment; perfluorooctanoic acid and methylene blue adsorption. Bioresour. Technol. 2021, 344, 126161. [Google Scholar] [CrossRef]
- Serafin, J.; Ouzzine, M.; Cruz, O.F.; Sreńscek-Nazzal, J.; Gómez, I.C.; Azar, F.-Z.; Mafull, C.A.R.; Hotza, D.; Rambo, C.R. Conversion of fruit waste-derived biomass to highly microporous activated carbon for enhanced CO2 capture. Waste Manag. 2021, 136, 273–282. [Google Scholar] [CrossRef]
- Ma, X.; Wu, Y.; Fang, M.; Liu, B.; Chen, R.; Shi, R.; Wu, Q.; Zeng, Z.; Li, L. In-situ activated ultramicroporous carbon materials derived from waste biomass for CO2 capture and benzene adsorption. Biomass Bioenergy 2022, 158, 106353. [Google Scholar] [CrossRef]
- Dombrowski, R.J.; Lastoskie, C.M.; Hyduke, D.R. The Horvath–Kawazoe method revisited. Colloids Surf. A Physicochem. Eng. Asp. 2001, 187–188, 23–39. [Google Scholar] [CrossRef]
- Puziy, A.; Poddubnaya, O.; Martinez-Alonso, A.; Suarez-Garcia, F.; Tascon, J. Synthetic carbons activated with phosphoric acid: I. Surface chemistry and ion binding properties. Carbon 2002, 40, 1493–1505. [Google Scholar] [CrossRef]
- Yacou, C.; Altenor, S.; Carene, B.; Gaspard, S. Chemical structure investigation of tropical Turbinaria turbinata seaweeds and its derived carbon sorbents applied for the removal of hexavalent chromium in water. Algal Res. 2018, 34, 25–36. [Google Scholar] [CrossRef]
- Oliveira, R.C.; Hammer, P.; Guibal, E.; Taulemesse, J.-M.; Garcia, O. Characterization of metal–biomass interactions in the lanthanum(III) biosorption on Sargassum sp. using SEM/EDX, FTIR, and XPS: Preliminary studies. Chem. Eng. J. 2014, 239, 381–391. [Google Scholar] [CrossRef]
- Wu, Y.; Li, B.; Feng, S.; Mi, X.; Jiang, J. Adsorption of Cr(VI) and As(III) on coaly activated carbon in single and binary systems. Desalination 2009, 249, 1067–1073. [Google Scholar] [CrossRef]
- Ozer, A.; Ozer, D. The Adsorption of Cr(VI) on Sulphuric Acid-Treated Wheat Bran. Environ. Technol. 2004, 25, 689–697. [Google Scholar] [CrossRef]
- Baral, S.; Das, S.; Chaudhury, G.R.; Swamy, Y.; Rath, P. Adsorption of Cr(VI) using thermally activated weed Salvinia cucullata. Chem. Eng. J. 2008, 139, 245–255. [Google Scholar] [CrossRef]
- A Dean, S.; Tobin, J.M. Uptake of chromium cations and anions by milled peat. Resour. Conserv. Recycl. 1999, 27, 151–156. [Google Scholar] [CrossRef]
- Selen, V.; Özer, D.; Özer, A. A Study on the Removal of Cr(VI) Ions by Sesame (Sesamum indicum) Stems Dehydrated with Sulfuric Acid. Arab. J. Sci. Eng. 2014, 39, 5895–5904. [Google Scholar] [CrossRef]
- Gamboa-Carballo, J.J.; Melchor-Rodríguez, K.; Hernández-Valdés, D.; Enriquez-Victorero, C.; Montero-Alejo, A.L.; Gaspard, S.; Jáuregui-Haza, U.J. Theoretical study of chlordecone and surface groups interaction in an activated carbon model under acidic and neutral conditions. J. Mol. Graph. Model. 2016, 65, 83–93. [Google Scholar] [CrossRef]
- Kekes, T.; Kolliopoulos, G.; Tzia, C. Hexavalent chromium adsorption onto crosslinked chitosan and chitosan/β-cyclodextrin beads: Novel materials for water decontamination. J. Environ. Chem. Eng. 2021, 9, 105581. [Google Scholar] [CrossRef]
- Aydın, Y.A.; Aksoy, N.D. Adsorption of chromium on chitosan: Optimization, kinetics and thermodynamics. Chem. Eng. J. 2009, 151, 188–194. [Google Scholar] [CrossRef]
- Shu, Y.; Ji, B.; Cui, B.; Shi, Y.; Wang, J.; Hu, M.; Luo, S.; Guo, D. Almond Shell-Derived, Biochar-Supported, Nano-Zero-Valent Iron Composite for Aqueous Hexavalent Chromium Removal: Performance and Mechanisms. Nanomaterials 2020, 10, 198. [Google Scholar] [CrossRef]
- Angerasa, F.T.; Kalifa, M.A.; Jembere, A.L.; Genet, M.B. Spent kaolin filter cake as an effective adsorbent for the removal of Hexavalent Chromium [Cr (VI)] from aqueous solution: Comparative study of wastewater treatment methods. South Afr. J. Chem. Eng. 2021, 38, 90–103. [Google Scholar] [CrossRef]
- da Costa, T.B.; da Silva, T.L.; Costa, C.S.D.; da Silva, M.G.C.; Vieira, M.G.A. Chromium adsorption using Sargassum filipendula algae waste from alginate extraction: Batch and fixed-bed column studies. Chem. Eng. J. Adv. 2022, 11, 100341. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef]
- Zhu, D.; Yang, H.; Chen, X.; Chen, W.; Cai, N.; Chen, Y.; Zhang, S.; Chen, H. Temperature-dependent magnesium citrate modified formation of MgO nanoparticles biochar composites with efficient phosphate removal. Chemosphere 2021, 274, 129904. [Google Scholar] [CrossRef]
- Ma, F.; Philippe, B.; Zhao, B.; Diao, J.; Li, J. Simultaneous adsorption and reduction of hexavalent chromium on biochar-supported nanoscale zero-valent iron (nZVI) in aqueous solution. Water Sci. Technol. 2020, 82, 1339–1349. [Google Scholar] [CrossRef]
- Shukla, S.; Kisku, G. Linear and Non-Linear Kinetic Modeling for Adsorption of Disperse Dye in Batch Process. Res. J. Environ. Toxicol. 2015, 9, 320–331. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Chen, M. Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. Bioresour. Technol. 2016, 214, 836–851. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, X.; Wang, D.; Wang, L.; Ma, F. Enhanced hexavalent chromium removal performance and stabilization by magnetic iron nanoparticles assisted biochar in aqueous solution: Mechanisms and application potential. Chemosphere 2018, 207, 50–59. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, Q.; Gao, B.; Li, M.; Liu, C.; Qiu, Y. Removal of hexavalent chromium by biochar supported nZVI composite: Batch and fixed-bed column evaluations, mechanisms, and secondary contamination prevention. Chemosphere 2018, 217, 85–94. [Google Scholar] [CrossRef]
- Qiao, K.; Tian, W.; Bai, J.; Zhao, J.; Du, Z.; Song, T.; Chu, M.; Wang, L.; Xie, W. Synthesis of floatable magnetic iron/biochar beads for the removal of chromium from aqueous solutions. Environ. Technol. Innov. 2020, 19, 100907. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, Y.; Xu, C.; Liu, P.; Lv, J.; Liu, Y.; Wang, Q. Removal mechanisms of aqueous Cr(VI) using apple wood biochar: A spectroscopic study. J. Hazard. Mater. 2020, 384, 121371. [Google Scholar] [CrossRef]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Prabhu, S.G.; Srinikethan, G.; Hegde, S. Pelletization of pristine Pteris vittata L. pinnae powder and its application as a biosorbent of Cd(II) and Cr(VI). SN Appl. Sci. 2019, 2, 132. [Google Scholar] [CrossRef]
- Pant, B.D.; Neupane, D.; Paudel, D.R.; Lohani, P.C.; Gautam, S.K.; Pokhrel, M.R.; Poudel, B.R. Efficient biosorption of hexavalent chromium from water by modified arecanut leaf sheath. Heliyon 2022, 8, e09283. [Google Scholar] [CrossRef]
- Masinga, T.; Moyo, M.; Pakade, V.E. Removal of hexavalent chromium by polyethyleneimine impregnated activated carbon: Intra-particle diffusion, kinetics and isotherms. J. Mater. Res. Technol. 2022, 18, 1333–1344. [Google Scholar] [CrossRef]
- Lin, H.; Han, S.; Dong, Y.; He, Y. The surface characteristics of hyperbranched polyamide modified corncob and its adsorption property for Cr(VI). Appl. Surf. Sci. 2017, 412, 152–159. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Song, J.; He, Q.; Hu, X.; Zhang, W.; Wang, C.; Chen, R.; Wang, H.; Mosa, A. Highly efficient removal of Cr(VI) and Cu(II) by biochar derived from Artemisia argyi stem. Environ. Sci. Pollut. Res. 2019, 26, 13221–13234. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Li, A. Eucalyptus sawdust derived biochar generated by combining the hydrothermal carbonization and low concentration KOH modification for hexavalent chromium removal. J. Environ. Manag. 2017, 206, 989–998. [Google Scholar] [CrossRef] [PubMed]








| Activated Carbons | SBET (m2/g) | Vmicro (cm3/g) | Vmeso (cm3/g) | Vmacro (cm3/g) | VTot (cm3/g) | Dp (nm) |
|---|---|---|---|---|---|---|
| SAC600 3/1 | 1695 | 0.727 | 0.773 | 0.339 | 1.838 | 4.34 |
| SBP10 | 1900 | 0.823 | 0.502 | 0.013 | 1.338 | 2.82 |
| Boehm Titration: Total Acidity and Total Basicity | ||
|---|---|---|
| Sample Name | Acidic Groups (mgeq H+/g) | Basic Groups (mgeq OH−/g) |
| SAC600 3/1 | 103.62 | 20.40 |
| SBP10 | 53.73 | 14.40 |
| Structure | Range (cm−1) | Peak (cm−1) | Probability | Assignment | References |
|---|---|---|---|---|---|
| RCH2CH3 (aliphatic sym.) | 3000–2850 | 2980 | Very possible | C-H stretching | [43,62] |
| RCH2CH3 (aliphatic asym.) | 3000–2850 | 2903 | Very possible | C-H stretching | [43,62] |
| Aromatic ring | 1571–1561 | 1569 | Very possible | C=C stretching | [43,62] |
| RCO-OH (carboxylic acids) | 1320–1000 | 1184; 1081; 1066 | Very possible | C-O stretching | [43,63] |
| RCOOR’ (esters) | 1320–1000 | 1184; 1081; 1066 | Possible | C-O stretching | [43,63] |
| Ar-OH (phenol) | 1205–1195 | 1184 | Very possible | C-O stretching | [43,62,63] |
| P=O (phosphine oxide) | 1200–1100 | 1184 | Possible | P=O vibration | [43,62,63] |
| P=O (phosphate) | 1200–1100 | 1184 | Very possible | P=O vibration | [43,62,63] |
| P=O (phosphate) | 1200–1100 | 1184 | Very possible | P=O vibration | [43,62,63] |
| C-O stretching | 1081 | 1081 | Very possible | C-O stretching | [55] |
| SAC600 3/1 | SBP10 | |||
|---|---|---|---|---|
| Elementary Composition of the Surface | % Atomic Concentration | Position BE (eV) | % Atomic Concentration | Position BE (eV) |
| C 1s | 83.3 | 284.655 | 92.99 | 284.03 |
| O 1s | 13.14 | 533.105 | 6.52 | 531.98 |
| N 1s | 3.23 | 400.155 | 0.49 | 133.18 |
| S 2p | 0.18 | 163.855 | ||
| P 2p | 0.15 | 133.655 | ||
| Functional Groups from C 1s | % Atomic Concentration | Position BE (eV) | % Atomic Concentration | Position BE (eV) |
| C=C, C-C, C-H | 67.23 | 284.596 | 80.88 | 284.400 |
| C-O | 21.09 | 285.739 | 7.45 | 286.250 |
| C=O | 7.43 | 286.904 | 6.19 | 287.950 |
| O-C=O | 4.24 | 288.381 | 5.48 | 289.990 |
| Activated Carbon | T (K) | ln(Keq) | ΔG° (KJ·mol−1) | ΔH° (KJ·mol−1) | ΔS° (J·mol−1·K−1) |
|---|---|---|---|---|---|
| SAC600 3/1 | 298.15 | 0.6878 | −1.70 | −21.85 | −67.3 |
| 308.15 | 0.5379 | −1.38 | |||
| 313.15 | 0.2292 | −0.60 |
| Model | Parameter | Activated Carbon | |
|---|---|---|---|
| SAC600 3/1 | SBP10 | ||
| Pseudo First-order Kinetics | qe (mg·g−1) | 17.91 | 5.23 |
| K1 (min–1) | 0.0017 | 0.0030 | |
| R2 | 0.8911 | 0.7209 | |
| Type 1—Pseudo Second-order Kinetics | qe (mg·g−1) | 46.97 | 36.26 |
| K2 (g/mg·min) | 0.0004 | 0.0026 | |
| R2 | 0.9973 | 0.9999 | |
| Type 2—Pseudo Second-order Kinetics | qe (mg·g−1) | 39.10 | 34.84 |
| K2 (g·mg−1·min−1) | 0.0044 | 0.0138 | |
| R2 | 0.6014 | 0.8307 | |
| Intraparticle Diffusion | Ci (mg·g−1) | 25.92 | 25.36 |
| Kip (mg·g−1·min−0.5) | 0.4830 | 0.3844 | |
| R2 | 0.5007 | 0.2463 | |
| Model | Parameter | Activated Carbon | |
|---|---|---|---|
| SAC600 3/1 | SBP10 | ||
| Langmuir | qs (mg·g−1) | 57.17 | 45.87 |
| K (L·g−1) | 0.1207 | 0.3015 | |
| R2 | 0.9676 | 0.8384 | |
| RSS | 75.49 | 435.66 | |
| Jovanovic | qs (mg·g−1) | 48.05 | 42.92 |
| K (L·g−1) | 0.1020 | 0.1547 | |
| R2 | 0.9516 | 0.7451 | |
| RSS | 112.73 | 687.09 | |
| Fowler | qs (mg·g−1) | 57.17 | 45.87 |
| K (L·g−1) | 0.1207 | 0.3015 | |
| χ | 0.00 | 0.00 | |
| R2 | 0.9676 | 0.8384 | |
| RSS | 75.49 | 435.66 | |
| Freundlich | K (L·g−1) | 12.0248 | 15.4435 |
| ν | 0.3809 | 0.3048 | |
| R2 | 0.9504 | 0.9749 | |
| RSS | 115.51 | 67.56 | |
| Jovanovic-Freundlich | qs (mg·g−1) | 54.54 | 58.94 |
| K (L·g−1) | 0.0744 | 0.0601 | |
| ν | 0.7014 | 0.4363 | |
| R2 | 0.9728 | 0.9459 | |
| RSS | 63.28 | 145.94 | |
| Fowler-Guggenheim/ Langmuir-Freundlich | qs (mg·g−1) | 64.27 | 37.80 |
| K (L·g−1) | 0.1552 | 1.6365 | |
| χ | 0.3509 | 0.5405 | |
| ν | 1.00 | 1.00 | |
| R2 | 0.9719 | 0.9627 | |
| RSS | 65.53 | 100.61 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez-Galvan, Y.; Minofar, B.; Futera, Z.; Francoeur, M.; Jean-Marius, C.; Brehm, N.; Yacou, C.; Jauregui-Haza, U.J.; Gaspard, S. Adsorption of Hexavalent Chromium Using Activated Carbon Produced from Sargassum ssp.: Comparison between Lab Experiments and Molecular Dynamics Simulations. Molecules 2022, 27, 6040. https://doi.org/10.3390/molecules27186040
Alvarez-Galvan Y, Minofar B, Futera Z, Francoeur M, Jean-Marius C, Brehm N, Yacou C, Jauregui-Haza UJ, Gaspard S. Adsorption of Hexavalent Chromium Using Activated Carbon Produced from Sargassum ssp.: Comparison between Lab Experiments and Molecular Dynamics Simulations. Molecules. 2022; 27(18):6040. https://doi.org/10.3390/molecules27186040
Chicago/Turabian StyleAlvarez-Galvan, Yeray, Babak Minofar, Zdeněk Futera, Marckens Francoeur, Corine Jean-Marius, Nicolas Brehm, Christelle Yacou, Ulises J. Jauregui-Haza, and Sarra Gaspard. 2022. "Adsorption of Hexavalent Chromium Using Activated Carbon Produced from Sargassum ssp.: Comparison between Lab Experiments and Molecular Dynamics Simulations" Molecules 27, no. 18: 6040. https://doi.org/10.3390/molecules27186040
APA StyleAlvarez-Galvan, Y., Minofar, B., Futera, Z., Francoeur, M., Jean-Marius, C., Brehm, N., Yacou, C., Jauregui-Haza, U. J., & Gaspard, S. (2022). Adsorption of Hexavalent Chromium Using Activated Carbon Produced from Sargassum ssp.: Comparison between Lab Experiments and Molecular Dynamics Simulations. Molecules, 27(18), 6040. https://doi.org/10.3390/molecules27186040