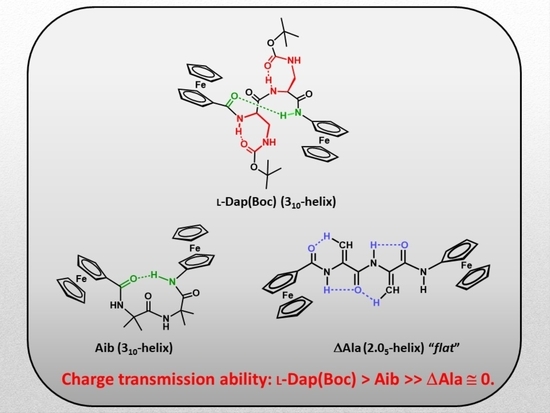
Helical versus Flat Bis-Ferrocenyl End-Capped Peptides: The Influence of the Molecular Skeleton on Redox Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Peptide Synthesis
2.2. Crystal State Conformational Analysis
2.3. Solution Conformational Analysis
2.4. Cyclic Voltammetry Analysis
2.5. Vis–MIR Chemical Oxidation
2.6. Circular Dichroism Analysis
3. Materials and Methods
3.1. Nuclear Magnetic Resonance
3.2. X-ray Diffraction
3.3. Cyclic Voltammetry
3.4. UV–Vis Analysis
3.5. FT–IR Analysis
3.6. Circular Dichroism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Moriuchi, T. Helical Chirality of Ferrocene Moieties in Cyclic Ferrocene-Peptide Conjugates. Eur. J. Inorg. Chem. 2022, 2022, e202100. [Google Scholar] [CrossRef]
- Tassinari, F.; Jayarathna, D.R.; Kantor-Uriel, N.; Davis, K.L.; Varade, V.; Achim, C.; Naaman, R. Chirality Dependent Charge Transfer Rate in Oligopeptides. Adv. Mater. 2018, 30, 1706423. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Tong, F.; Zhang, W.; Zhou, Y.; He, S.Q.; Xie, R.; Lei, T.; Wang, Y.S.; Peng, S.J.; Li, Z.F.; et al. Self-Delivered Supramolecular Nanomedicine with Transformable Shape for Ferrocene-Amplified Photodynamic Therapy of Breast Cancer and Bone Metastases. Adv. Funct. Mater. 2021, 31, 2104645. [Google Scholar] [CrossRef]
- Gómez, J.; Sierra, D.; Ojeda, C.; Thavalingam, S.; Miller, R.; Guzmán, F.; Metzler-Nolte, N. Solid-phase synthesis and evaluation of linear and cyclic ferrocenoyl/ruthenocenoyl water-soluble hexapeptides as potential antibacterial compounds. J. Biol. Inorg. Chem. 2021, 26, 599–615. [Google Scholar] [CrossRef]
- Gurunarayanan, V.; Ramapanicker, R. Amphiphilic conjugates of ferrocene with amino acids and peptides: Design, synthesis, and studies on their aggregation behavior. J. Pep. Sci. 2021, 27, e3332. [Google Scholar] [CrossRef]
- Costa, N.C.S.; Piccoli, J.P.; Santos-Filho, N.A.; Clementino, L.C.; Fusco-Almeida, A.M.; De Annunzio, S.R.; Fontana, C.R.; Verga, J.B.; Eto, S.F.; Pizauro-Junior, J.M.; et al. Antimicrobial Activity of RP-1 Peptide Conjugate with Ferrocene Group. PLoS ONE 2020, 15, e0228740. [Google Scholar] [CrossRef]
- Peter, S.; Aderibigbe, B.A. Ferrocene-Based Compounds with Antimalaria/Anticancer Activity. Molecules 2019, 24, 3604. [Google Scholar] [CrossRef]
- Falcone, N.; Kraatz, H.-B. Ferrocene Peptide-based Supramolecular Gels: Current Trends and Applications. In Advances in Bioorganometallic Chemistry; Hirao, T., Moriuchi, T., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Chapter 3; pp. 57–74. [Google Scholar]
- Yu, J.; Horsley, J.R.; Abell, A.D. Peptides as Bio-Inspired Electronic Materials: An Electrochemical and First-Principles Perspective. Acc. Chem. Res. 2018, 51, 2237–2246. [Google Scholar] [CrossRef]
- Albada, B.; Metzler-Nolte, N. Highly Potent Antibacterial Organometallic Peptide Conjugates. Acc. Chem. Res. 2017, 50, 2510–2518. [Google Scholar] [CrossRef]
- Hirao, T.; Moriuchi, M.; Groß, A. Functionalized Redox Systems. Synthetic Reactions and Design of π- and Bio-Conjugates. In Functionalized Redox Systems; Chapter 4 Bioconjugates to Induce Chirality Organization; Hirao, T., Ed.; Springer: Tokyo, Japan, 2015; pp. 111–150. [Google Scholar]
- Adhikariab, B.; Kraatz, H.-B. Redox-triggered changes in the self-assembly of a ferrocene–peptide conjugated. Chem. Commun. 2014, 50, 5551–5553. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, S.; Anzai, J.-I. Recent Progress in Ferrocene-Modified Thin Films and Nanoparticles for Biosensors. Materials 2013, 6, 5742. [Google Scholar] [CrossRef] [PubMed]
- Zuccarello, L.; Barbosa, C.; Todorovic, S.; Silveira, C.M. Electrocatalysis by Heme Enzymes—Applications in Biosensing. Catalysts 2021, 11, 218. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, P.; Jin, X.; Wang, Y.; Yuan, H.; Zhu, X. Enzymatic biofuel cells based on protein engineering: Recent advances and future prospects. Biomater. Sci. 2020, 8, 5230–5524. [Google Scholar] [CrossRef]
- Yu, S.Y.; Myung, N.V. Recent Advances in the Direct Electron Transfer-Enabled Enzymatic Fuel Cells. Front. Chem. 2021, 8, 620153. [Google Scholar] [CrossRef]
- Zhang, L.; Yagnik, G.; Peng, J.; Wang, J.; Xu, H.H.; Hao, Y.; Liu, Y.-N.; Zhou, F. Kinetic Studies of Inhibition of the Aβ(1–42) Aggregation Using a Ferrocene-tagged β-Sheet Breaker Peptide. Anal. Biochem. 2013, 434, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Afrasiabi, R.; Kraatz, H.B. Stimuli-Responsive Supramolecular Gelation in Ferrocene–Peptide Conjugates. Chem. Eur. J. 2013, 19, 17296–17300. [Google Scholar] [CrossRef]
- Martić, S.; Labib, M.; Shipman, P.-O.; Kraatz, H.-B. Ferrocene-peptido conjugates: From synthesis to sensory applications. Dalton Trans. 2011, 40, 7264–7290. [Google Scholar] [CrossRef]
- Moriuchi, T.; Kikushima-Honda, N.; Ohmura, S.D.; Hirao, T. Design and characterization of ferrocene–peptide–oligoaniline conjugates. Tetrahedron Lett. 2010, 51, 4530–4533. [Google Scholar] [CrossRef]
- Okamoto, S.; Morita, T.; Kimura, S. Electron Transfer through a Self-Assembled Monolayer of a Double-Helix Peptide with Linking the Terminals by Ferrocene. Langmuir 2009, 25, 3297–3304. [Google Scholar] [CrossRef]
- Adachi, T.; Kitazumi, Y.; Shirai, O.; Kano, K. Direct Electron Transfer-Type Bioelectrocatalysis of Redox Enzymes at Nanostructured Electrodes. Catalysts 2020, 10, 236. [Google Scholar] [CrossRef] [Green Version]
- Donoli, A.; Marcuzzo, V.; Moretto, A.; Toniolo, C.; Cardena, R.; Bisello, A.; Santi, S. Charge Mapping in 310-Helical Peptide Chains by Oxidation of the Terminal Ferrocenyl Group. Org. Lett. 2011, 13, 1282–1285. [Google Scholar] [CrossRef] [PubMed]
- Biondi, B.; Bisello, A.; Cardena, R.; Schiesari, R.; Facci, M.; Cerveson, L.; Rancan, M.; Formaggio, F.; Santi, S. Conformational Analysis and Through-Chain Charge Propagation in Ferrocenyl-Conjugated Homopeptides of 2,3-Diaminopropionic acid (Dap). Eur. J. Inorg. Chem. 2022, 2022, e202100. [Google Scholar] [CrossRef]
- Biondi, B.; Bisello, A.; Cardena, R.; Schiesari, R.; Cerveson, L.; Facci, M.; Rancan, M.; Formaggio, F.; Santi, S. Flat, Ferrocenyl-Conjugated Peptides: A Combined Electrochemical and Spectroscopic Study. ChemElectroChem 2021, 8, 2693–2700. [Google Scholar] [CrossRef]
- Donoli, A.; Marcuzzo, V.; Moretto, M.; Crisma, M.; Toniolo, C.; Cardena, R.; Bisello, A.; Santi, S. New bis-Ferrocenyl End-Capped Peptides: Synthesis and ChargeTransfer Properties. Pept. Sci. 2012, 100, 14–24. [Google Scholar] [CrossRef]
- Santi, S.; Bisello, A.; Cardena, R.; Donoli, A. Key Multi(Ferrocenyl) Complexes in the Interplay between Electronic Coupling and Electrostatic Interaction. Dalton Trans. 2005, 44, 5234–5257. [Google Scholar] [CrossRef]
- Santi, S.; Bisello, A.; Cardena, R.; Tomelleri, S.; Schiesari, R.; Biondi, B.; Crisma, M.; Formaggio, F. Flat, Cα,β-Didehydroalanine Foldamers with Ferrocene Pendants: Assessing the Role of α-Peptide Dipolar Moments. ChemPlusChem 2021, 86, 723–730. [Google Scholar] [CrossRef]
- Nuskol, M.; Studen, B.; Meden, A.; Kodrin, I.; Semenčic, M.C. Tight turn in dipeptide bridged ferrocenes: Synthesis, X-ray structural, theoretical and spectroscopic studies. Polyhedron 2019, 161, 137–144. [Google Scholar] [CrossRef]
- Shah, A.; Adhikari, B.S.; Martic Munir, A.; Shahzad, S.; Ahmad, K.; Kraatz, H.-B. Electron transfer in peptides. Chem. Soc. Rev. 2015, 44, 1015–1027. [Google Scholar] [CrossRef]
- Amdursky, N. Electron Transfer across Helical Peptides. ChemPlusChem 2015, 80, 1075–1095. [Google Scholar] [CrossRef]
- Antonello, S.; Formaggio, F.; Moretto, A.; Toniolo, C.; Maran, F. Anomalous Distance Dependence of Electron Transfer across Peptide Bridges. J. Am. Chem. Soc. 2003, 125, 2874–2875. [Google Scholar] [CrossRef]
- Improta, R.; Antonello, S.; Formaggio, F.; Maran, F.; Rega, N.; Barone, V. Understanding Electron Transfer across Negatively-Charged Aib Oligopeptides. J. Phys. Chem. B 2005, 109, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Polo, F.; Antonello, S.; Formaggio, F.; Toniolo, C.; Maran, F. Evidence Against the Hopping Mechanism as an Important Electron Transfer Pathway for Conformationally Constrained Oligopeptide. J. Am. Chem. Soc. 2005, 127, 492–493. [Google Scholar] [CrossRef]
- Gatto, E.; Stella, L.; Formaggio, F.; Toniolo, C.; Lorenzelli, L.; Venanzi, M. Electroconductive and photocurrent generation properties of self-assembled monolayers formed by functionalized, conformationally-constrained peptides on gold electrodes. J. Pept. Sci. 2008, 14, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Gatto, E.; Stella, L.; Baldini, C.; Toniolo, C.; Formaggio, F.; Venanzi, M. Photocurrent generation in peptide-based self-assembled monolayers on gold electrodes. Superlattices Microstruct. 2009, 46, 34–39. [Google Scholar] [CrossRef]
- Arikuma, Y.; Nakayama, H.; Morita, T.; Kimura, S. Langmuir Ultra-Long-Range Electron Transfer through a Self-Assembled Monolayer on Gold Composed of 120-Å-Long α-Helices. Langmuir 2011, 27, 1530–1535. [Google Scholar] [CrossRef]
- Garbuio, L.; Antonello, S.; Guryanov, I.; Li, Y.J.; Ruzzi, M.; Turro, N.J.; Maran, F. Effect of Orientation of the Peptide-Bridge Dipole Moment on the Properties of Fullerene–Peptide–Radical Systems. J. Am. Chem. Soc. 2012, 134, 10628–10637. [Google Scholar] [CrossRef]
- Mehlhose, S.; Frenkel, N.; Uji, H.; Hölzel, S.; Müntze, V.; Stock, D.; Neugebauer, S.; Dadgar, A.; Abuillan, W.; Eickhoff, M.; et al. Flexible Modulation of Electronic Band Structures of Wide Band Gap GaN Semiconductors Using Bioinspired, Nonbiological Helical Peptides. Adv. Funct. Mater. 2018, 28, 1704034. [Google Scholar] [CrossRef]
- Zuliani, C.; Formaggio, F.; Scipionato, L.; Toniolo, C.; Antonello, S.; Maran, F. Insights into the Distance Dependence of Electron Transfer through Conformationally Constrained Peptides. ChemElectroChem 2020, 7, 1225–1237. [Google Scholar] [CrossRef]
- Fox, M.A.; Galoppini, E.J. Electric Field Effects on Electron Transfer Rates in Dichromophoric Peptides: The Effect of Helix Unfolding. J. Am. Chem. Soc. 1997, 119, 5277–5285. [Google Scholar] [CrossRef]
- Yasutomi, S.; Morita, T.; Imanishi, Y.; Kimura, S. A Molecular Photodiode System That Can Switch Photocurrent Direction. Science 2004, 304, 1944–1947. [Google Scholar] [CrossRef]
- Yasutomi, S.; Morita, T.; Kimura, S. pH-Controlled Switching of Photocurrent Direction by Self-Assembled Monolayer of Helical Peptides. J. Am. Chem. Soc. 2005, 127, 14564–14565. [Google Scholar] [CrossRef] [PubMed]
- Gatto, E.; Porchetta, A.; Stella, L.; Guryanov, I.; Formaggio, F.; Toniolo, C.; Kaptein, B.; Broxterman, Q.B.; Venanzi, M. Conformational Effects on the Electron-Transfer Efficiency in Peptide Foldamers Based on α,α-Disubstituted Glycyl Residues. Chem. Biodivers. 2008, 5, 1263–1278. [Google Scholar] [CrossRef] [PubMed]
- Gatto, E.; Caruso, M.; Porchetta, A.; Toniolo, C.; Formaggio, F.; Crisma, M.; Venanzi, M. Conformational Effects on the Electron-Transfer Efficiency in Peptide Foldamers Based on α,α -Disubstituted Glycyl Residues. J. Pept. Sci. 2011, 17, 24–131. [Google Scholar] [CrossRef] [PubMed]
- Gatto, E.; Venanzi, M. The Impervious Route to Peptide-Based Dye-Sensitized Solar Cells. Isr. J. Chem. 2015, 55, 671–681. [Google Scholar] [CrossRef]
- Gobbo, P.; Antonello, S.; Guryanov, I.; Polo, F.; Soldà, A.; Zen, F.; Maran, F. Dipole Moment Effect on the Electrochemical Desorption of Self-Assembled Monolayers of 310-Helicogenic Peptides on Gold. ChemElectroChem 2016, 3, 2063–2070. [Google Scholar] [CrossRef]
- Viso, A.; de la Pradilla, R.F.; García, A.; Flores, A. α,β-Diamino Acids: Biological Significance and Synthetic Approaches. Chem. Rev. 2005, 105, 3167–3196. [Google Scholar] [CrossRef]
- Meierhenrich, U.J.; Muñoz Caro, G.M.; Bredehöft, J.H.; Jessberger, E.K.; Thiemann, W.H.-P. Identification of diamino acids in the Murchison meteorite. Proc. Natl. Acad. Sci. USA 2004, 101, 9182–9186. [Google Scholar] [CrossRef]
- Delatouche, R.; Durini, M.; Civera, M.; Belvisi, L.; Piarulli, U. Foldamers of bifunctional diketopiperazines displaying a β-bend ribbon structure. Tetrahedron Lett. 2010, 51, 4278–4280. [Google Scholar] [CrossRef]
- Musumeci, D.; Roviello, V.; Roviello, G.N. DNA- and RNA-binding ability of oligoDapT, a nucleobase-decorated peptide, for biomedical applications. Int. J. Nanomed. 2018, 13, 2613–2629. [Google Scholar] [CrossRef]
- Arokianathan, J.F.; Ramya, K.A.; Deshpande, A.P.; Leemarose, A.; Shanmugam, G. Supramolecular organogel based on di-Fmoc functionalized unnatural amino acid: An attempt to develop a correlation between molecular structure and ambidextrous gelation. Colloids Surf. A 2021, 618, 126430. [Google Scholar] [CrossRef]
- McNulty, J.C.; Thompson, D.A.; Carrasco, M.R.; Millhauser, G.L. Dap-SL: A new site-directed nitroxide spin labeling approach for determining structure and motions in synthesized peptides and proteins. FEBS Lett. 2002, 529, 243–248. [Google Scholar] [CrossRef]
- Inoue, G.; Toyohara, D.; Mori, T.; Muraoka, T. Critical Side Chain Effects of Cell-Penetrating Peptides for Transporting Oligo Peptide Nucleic Acids in Bacteria. ACS Appl. Bio Mater. 2021, 4, 3462–3468. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, S.; York, E.J.; Stewart, J.M.; Baldwin, R.L.J. Helix Propensities of Basic Amino Acids Increase with the Length of the Side-chain. Mol. Biol. 1996, 257, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Deechongkit, S.; Kennedy, R.J.; Tsang, K.Y.; Renold, P.; Kemp, D.S. An amino acid that controls polypeptide helicity: β-amino alanine, the first strongly stabilizing C-terminal helix stop signal. Tetrahedron Lett. 2000, 41, 9679–9683. [Google Scholar] [CrossRef]
- Houlton, A.; Isaac, C.J.; Gibson, A.E.; Horrocks, B.R.; Clegg, W.; Elsegood, M.R.J. Synthesis, structure and redox properties of ferrocenylmethylnucleobases. J. Chem. Soc. Dalton Trans. 1999, 18, 3229–3234. [Google Scholar] [CrossRef]
- Kraatz, H.-B. Ferrocene-Conjugates of Amino Acids, Peptides and Nucleic Acids. J. Inorg. Organomet. Polym. Mater. 2005, 15, 83–106. [Google Scholar] [CrossRef]
- Venkatachalam, C.M. Stereochemical criteria for polypeptides and proteins. V. Conformation of a system of three linked peptide units. Biopolymers 1968, 6, 1425–1436. [Google Scholar] [CrossRef]
- Taylor, R.; Kennard, O.; Versichel, W. The geometry of the N–H⋯O=C hydrogen bond. 3. Hydrogen-bond distances and angles. Acta Crystallogr. B 1984, 40, 280–288. [Google Scholar] [CrossRef]
- Görbitz, C.H. β Turns, water cage formation and hydrogen bonding in the structures of L-valyl-L-phenylalanine. Acta Crystallogr. B 1989, 45, 390–395. [Google Scholar] [CrossRef]
- Torshin, I.Y.; Weber, I.T.; Harrosin, R.W. Geometric criteria of hydrogen bonds in proteins and identification of “bifurcated” hydrogen bonds. Protein Eng. 2002, 15, 359–363. [Google Scholar] [CrossRef] [Green Version]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford Univeraity Press: Oxford, UK, 1999; pp. 108–113. [Google Scholar]
- Metzler-Nolte, N.; Salmain, M. Ferrocenes: Ligands, Materials and Biomolecules; Štěpnička, P., Ed.; Wiley: Chichester, UK, 2008; pp. 499–639. [Google Scholar]
- Kraatz, H.-B.; Leek, D.M.; Houmam, A.; Enright, G.D.; Lusztyk, J.; Wayner, D.D. The ferrocene moiety as a structural probe: Redox and structural properties of ferrocenoyl-oligoprolines Fc–Pron–OBzl (n = 1–4) and Fc–Pro2–Phe–OBzl. J. Organomet. Chem. 1999, 589, 38–49. [Google Scholar] [CrossRef]
- Peggion, C.; Moretto, A.; Formaggio, F.; Crisma, M.; Toniolo, C. Multiple, Consecutive, Fully-Extended 2.0(5)-Helix Peptide Conformation. Multiple, consecutive, fully-extended 2.05-helix peptide conformation. Biopolymers 2013, 100, 621–636. [Google Scholar] [CrossRef] [PubMed]
- Crisma, M.; Formaggio, F.; Alemán, C.; Torras, J.; Ramakrishnan, C.; Kalmankar, N.; Balaram, P.; Toniolo, C. The fully-extended conformation in peptides and proteins. Pept. Sci. 2018, 110, e23100. [Google Scholar] [CrossRef]
- Guryanov, I.; Moretto, A.; Campestrini, S.; Broxterman, Q.B.; Kaptein, B.; Peggion, C.; Formaggio, F.; Toniolo, C. Turn and helical peptide spacers: Combined distance and angular dependencies in the exciton-coupled circular dichroism of intramolecularly interacting bis-porphyrins. Biopolymers 2006, 82, 482–490. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Carrozzini, B.; Cascarano, G.L.; Cuocci, C.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.; Polidori, G. Crystal structure determination and refinement via SIR2014. J. Appl. Crystallogr. 2015, 48, 306–309. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Amatore, C.; Lefrou, C.; Pflüger, F. On-line compensation of ohmic drop in submicrosecond time resolved cyclic voltammetry at ultramicroelectrodes. J. Electroanal. Chem. 1989, 270, 43–59. [Google Scholar] [CrossRef]













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santi, S.; Biondi, B.; Cardena, R.; Bisello, A.; Schiesari, R.; Tomelleri, S.; Crisma, M.; Formaggio, F. Helical versus Flat Bis-Ferrocenyl End-Capped Peptides: The Influence of the Molecular Skeleton on Redox Properties. Molecules 2022, 27, 6128. https://doi.org/10.3390/molecules27186128
Santi S, Biondi B, Cardena R, Bisello A, Schiesari R, Tomelleri S, Crisma M, Formaggio F. Helical versus Flat Bis-Ferrocenyl End-Capped Peptides: The Influence of the Molecular Skeleton on Redox Properties. Molecules. 2022; 27(18):6128. https://doi.org/10.3390/molecules27186128
Chicago/Turabian StyleSanti, Saverio, Barbara Biondi, Roberta Cardena, Annalisa Bisello, Renato Schiesari, Silvia Tomelleri, Marco Crisma, and Fernando Formaggio. 2022. "Helical versus Flat Bis-Ferrocenyl End-Capped Peptides: The Influence of the Molecular Skeleton on Redox Properties" Molecules 27, no. 18: 6128. https://doi.org/10.3390/molecules27186128
APA StyleSanti, S., Biondi, B., Cardena, R., Bisello, A., Schiesari, R., Tomelleri, S., Crisma, M., & Formaggio, F. (2022). Helical versus Flat Bis-Ferrocenyl End-Capped Peptides: The Influence of the Molecular Skeleton on Redox Properties. Molecules, 27(18), 6128. https://doi.org/10.3390/molecules27186128