Utilization of a Novel Immunofluorescence Instrument Prototype for the Determination of the Herbicide Glyphosate
Abstract
:1. Introduction
1.1. Glyphosate Herbicide Active Ingredient as an Environmental Pollutant
1.2. Analytical Methods for the Determination of Glyphosate
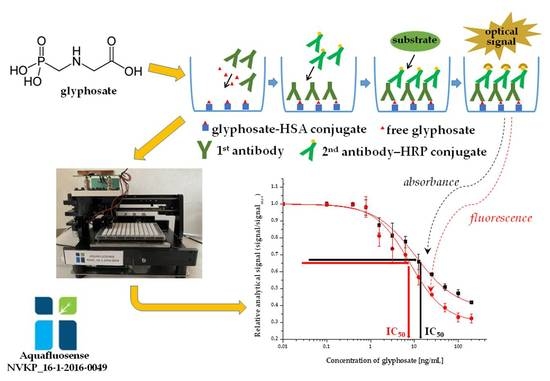
2. Results and Discussion
2.1. Enzyme-Linked Immunosorbent Assay Development for Glyphosate
2.1.1. Immunoassay Optimization
2.1.2. Cross-Reactivity of the Glyphosate-Specific Antibody
2.2. Application of the ELFIA for Glyphosate in Environmental and Biological Samples
2.2.1. Surface Water and Soil Samples
2.2.2. Biological Samples
3. Materials and Methods
3.1. Materials and Reagents
3.2. Instrumentation
3.3. Enzyme-Linked Immunosorbent Assay
3.3.1. Hapten Synthesis and Conjugation
3.3.2. Immunoassay
3.4. Application of Immunoassay in Surface Water, Soil and Plant Tissues
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef]
- Bhatt, P.; Bhandari, G.; Bilal, M. Occurrence, toxicity impacts and mitigation of emerging micropollutants in the aquatic environments: Recent tendencies and perspectives. J. Environ. Chem. Eng. 2022, 10, 107598. [Google Scholar] [CrossRef]
- Ziylan-Yavas, A.; Santos, D.; Flores, E.M.M.; Ince, N.H. Pharmaceuticals and personal care products (PPCPs): Environmental and public health risks. Environ. Prog. Sustain. Energy 2022, 41, e13821. [Google Scholar] [CrossRef]
- Székács, A.; Mörtl, M.; Darvas, B. Monitoring pesticide residues in surface and ground water in Hungary—Surveys in 1990–2015. J. Chem. 2015, 2015, 717948. [Google Scholar] [CrossRef] [Green Version]
- Lundqvist, J.; von Brömssen, C.; Rosenmai, A.K.; Ohlsson, Å.; Le Godec, T.; Jonsson, O.; Kreuger, J.; Oskarsson, A. Assessment of pesticides in surface water samples from Swedish agricultural areas by integrated bioanalysis and chemical analysis. Environ. Sci. Eur. 2019, 31, 53. [Google Scholar] [CrossRef] [Green Version]
- Campanale, C.; Massarelli, C.; Losacco, D.; Bisaccia, D.; Triozzi, M.; Uricchio, V.F. The monitoring of pesticides in water matrices and the analytical criticalities: A review. Trends Anal. Chem. 2021, 144, 116423. [Google Scholar]
- Battaglin, W.A.; Meyer, M.T.; Kuivila, K.M.; Dietze, J.E. Glyphosate and Its Degradation Product AMPA Occur Frequently and Widely in U.S. Soils, Surface Water, Groundwater, and Precipitation. JAWRA 2014, 50, 275–290. [Google Scholar]
- Székács, A.; Darvas, B. Re-registration Challenges of Glyphosate in the European Union. Front. Environ. Sci. 2018, 6, 78. [Google Scholar] [CrossRef] [Green Version]
- Carles, L.; Gardon, H.; Joseph, L.; Sanchís, J.; Farré, M.; Artigas, J. Meta-analysis of glyphosate contamination in surface waters and dissipation by biofilms. Environ. Int. 2019, 124, 284–293. [Google Scholar] [CrossRef]
- Vereecken, H. Mobility and leaching of the glyphosate: A review. Pest. Manag. Sci. 2005, 61, 1139–1151. [Google Scholar]
- Székács, A.; Darvas, B. Forty years with glyphosate. In Herbicides—Properties, Synthesis and Control of Weeds; Hasaneen, M.N.A.E.-G., Ed.; InTech: Rijeka, Croatia, 2012; pp. 247–284. [Google Scholar]
- Hanke, I.; Wittmer, I.; Bischofberger, S.; Stamm, C.; Singer, H. Relevance of urban glyphosate use for surface water quality. Chemosphere 2010, 81, 3. [Google Scholar] [CrossRef] [PubMed]
- Krüger, M.; Schrödl, W.; Neuhaus, J.; Shehata, A.A. Field investigations of glyphosate in urine of Danish dairy cows. J. Environ. Anal. Toxicol. 2013, 3, 5. [Google Scholar]
- Zoller, O.; Rhyn, P.; Zarn, J.A.; Dudler, V. Urine glyphosate level as a quantitative biomarker of oral exposure. Int. J. Hyg. Environ. Health 2020, 228, 113526. [Google Scholar] [CrossRef] [PubMed]
- Grau, D.; Grau, N.; Gascuel, Q.; Paroissin, C.; Stratonovitch, C.; Lairon, D.; Devault, D.A.; Di Cristofaro, J. Quantifiable urine glyphosate levels detected in 99% of the French population, with higher values in men, in younger people, and in farmers. Environ. Sci. Pollut. Res. 2022, 29, 32882–32893. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Ito, Y.; Taga, A.; Nomura, Y.; Yamamoto, A.; Chinaka, S.; Suzuki, K.; Yamashita, T.; Kemmei, T.; Hayakawa, K. A fast and simple analysis of glyphosate in tea beverages by capillary electrophoresis with on-line copper(II)-Glyphosate complex formation. J. Health Sci. 2008, 54, 602–606. [Google Scholar] [CrossRef] [Green Version]
- Qian, K.; Tang, T.; Shi, T.; Wang, F.; Li, J.; Cao, Y. Residue determination of glyphosate in environmental water samples with high-performance liquid chromatography and UV detection after derivatization with 4-chloro-3,5-dinitrobenzotrifluoride. Anal. Chim. Acta 2009, 635, 222–226. [Google Scholar] [CrossRef]
- Songa, E.A.; Arotiba, O.A.; Owino, J.H.O.; Jahed, N.; Baker, P.G.L.; Iwuoha, E.I. Electrochemical detection of glyphosate herbicide using horseradish peroxidise immobilized on sulfonated polymer matrix. Bioelectrochemistry 2009, 75, 117–123. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Hyne, R.V.; Desseile, K.L. An amperometric method for the detection of amitrole, glyphosate and its aminomethyl-phosphonic acid metabolite in environmental waters using passive samplers. Anal. Chim. Acta 2010, 675, 125–131. [Google Scholar] [CrossRef]
- Stalikas, C.D.; Konidari, C.N. Analytical methods to determine phosphonic and amino acid group-containing pesticides. J. Chromatogr. A 2001, 907, 1–19. [Google Scholar] [CrossRef]
- Kataoka, H.; Ryu, S.; Sakiyama, N.; Makita, M. Simple and rapid determination of the herbicides glyphosate and glufosinate in river water, soil and carrot samples by gas chromatography with flame photometric detection. J. Chromatogr. A 1996, 726, 253–258. [Google Scholar] [CrossRef]
- Tseng, S.H.; Lo, Y.W.; Chang, P.C.; Chou, S.S.; Chang, H.M. Simultaneous quantification of glyphosate, glufosinate, and their major metabolites in rice and soybean sprouts by gas chromatography with pulsed flame photometric detector. J. Agric. Food Chem. 2004, 52, 4057–4063. [Google Scholar] [CrossRef] [PubMed]
- Durán-Merás, I.; Díaz, T.G.; Franco, M.A. Simultaneous fluorimetric determination of glyphosate and its metabolite, aminomethylphosphonic acid, in water previous derivatization with NBD-Cl and by partial least squares calibration (PLS). Talanta 2005, 65, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Freuze, I.; Jadas-Hecart, A.; Royer, A.; Communal, P.Y. Communal, Influence of complexation phenomena with multivalent cations on the analysis of glyphosate and aminomethyl phosphonic acid in water. J. Chromatogr. A 2007, 1175, 197–206. [Google Scholar] [CrossRef]
- Vreeken, R.J.; Speksnijader, P.; Bobeldijk-Pastorova, I.; Noij, T.H.M. Selective analysis of the herbicides glyphosate and aminomethylphosphonic acid in water by online solid-phase extraction-high-performance liquid chromatography-electrospray ionization mass spectrometry. J. Chromatogr. A 1998, 794, 187–199. [Google Scholar] [CrossRef]
- Ibáñez, M.; Pozo, O.J.; Sancho, J.V.; López, F.J.; Hernández, F. Re-evaluation of glyphosate determination in water by liquid chromatography coupled to electrospray tandem mass spectrometry. J. Chromatogr. A 2006, 1134, 51–55. [Google Scholar] [PubMed]
- Glass, R.L. Colorimetric determination of glyphosate in water after oxidation to orthophosphate. Anal. Chem. 1981, 53, 921–923. [Google Scholar] [CrossRef]
- Jan, R.; Shah, J.; Muhammad, M.; Ara, B. Glyphosate herbicide residue determination in samples of environmental importance using spectrophotometric method. J. Hazard. Mater. 2009, 169, 742–745. [Google Scholar] [CrossRef]
- Waiman, C.V.; Avena, M.J.; Garrido, M.; Band, B.F.; Zanini, G.P. A simple and rapid spectrophotometric method to quantify the herbicide glyphosate in aqueous media. Application to adsorption isotherms on soils and goethite. Geoderma 2012, 170, 154–158. [Google Scholar] [CrossRef]
- Albers, C.N.; Banta, G.T.; Hansen, P.E.; Jacobsen, O.S. The influence of organic matter on sorption and fate of glyphosate in soil—Comparing different soils and humic substances. Environ. Pollut. 2009, 157, 2865–2870. [Google Scholar] [CrossRef]
- Aquafluosense. Development of a Modular, Direct and Immunofluorimetry as Well as Plasma Spectroscopy Based Detector and Instrument Family for In Situ, Complex Water Quality Monitoring, and Application Studies. Available online: http://aquafluosense.hu (accessed on 1 September 2022).
- Rodbard, D.; Hutt, D.M. Statistical analysis of radioimmunoassays and immunoradiometric (labeled antibody) assays: A generalized, weighted, iterative, least-squares method for logistic curve fitting. In Proceedings of the Symposium on RIA and Related Procedures in Medicine, Internationaé Atomic Energy Agency, Istanbul, Turkey, 10 September 1973; Volume 1, pp. 165–189. [Google Scholar]
- European Parliament and Council. Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption. Off. J. Eur. Union 2020, L435, 1–62. [Google Scholar]
- Gémes, B.; Takács, E.; Gádoros, P.; Barócsi, A.; Kocsányi, L.; Lenk, S.; Csákányi, A.; Kautny, S.; Domján, L.; Szarvas, G.; et al. Development of an Immunofluorescence Assay Module for Determination of the Mycotoxin Zearalenone in Water. Toxins 2021, 13, 182. [Google Scholar] [CrossRef] [PubMed]
- Thermo Fisher Scientific Inc. Instructions. QuantaRed™ Enhanced Chemifluorescent HRP Substrate; Thermo Fisher Scientific Inc.: Rockford, lL, USA, 2008; pp. 1–3. Available online: https://assets.thermofisher.com/TFS-Assets%2FLSG%2Fmanuals%2FMAN0011632_QuantaRed_Enhan_ChemiFluores_HRP_Subs_UG.pdf (accessed on 1 September 2022).




| Equation for Fitting: 1 Adjusted R2: 0.9859 (absorbance) 0.9895 (fluorescence) | ||
|---|---|---|
| Parameter | Normalized Value ± SD 2 | |
| Absorbance | A1 | 1.01 ± 0.01 |
| A2 | 0.38 ± 0.01 | |
| x0 | 10.75 ± 1.07 | |
| p | 0.94 ± 0.11 | |
| Fluorescence | A1 | 1.01 ± 0.01 |
| A2 | 0.30 ± 0.02 | |
| x0 | 7.94 ± 0.95 | |
| p | 1.10 ± 0.12 | |
| Intra-Assay | Inter-Assay | ||
|---|---|---|---|
| Average (ng/mL) | CV% | Average (ng/mL) | CV% |
| 0.28 | 25.1 | 0.45 | 15.0 |
| 1.04 | 14.4 | 1.43 | 9.2 |
| 3.74 | 8 | 4.27 | 6.9 |
| 11.84 | 8.7 | 12.14 | 7.7 |
| Compound | Nominal Concentration (ng/mL) | Detected Concentration (ng/mL) | Detected/Nominal Concentration (%) |
|---|---|---|---|
| glyphosate * | 100 | 99.3 ± 0.8 | 100 |
| 50 | 50.4 ± 1.1 | 100 | |
| AMPA * | 6700 | <0.1 | <0.0015 |
| 100 | <0.1 | <0.01 | |
| PMIDA * | 6700 | 0.89 | 0.013 |
| 1650 | 0.31 | 0.018 | |
| iminodiacetic acid | 100 | <0.1 | <0.01 |
| sarcosine * | 100 | <0.1 | <0.01 |
| glycine | 6700 | <0.1 | <0.0015 |
| 100 | <0.1 | <0.01 | |
| acetylglycine | 100 | <0.1 | <0.01 |
| Nominal Phosphate Concentration (mM) | Detected Glyphosate Concentration (ng/mL) | Detected/Nominal Concentration (%) |
|---|---|---|
| 175.0 | 17.6 | <0.0015 (6.0 × 10−5) |
| 87.5 | 8.40 | <0.0015 (5.7 × 10−5) |
| 43.8 | 4.10 | <0.0015 (5.5 × 10−5) |
| 21.9 | 2.26 | <0.0015 (6.1 × 10−5) |
| average | <0.0015 (5.8 × 10−5) |
| Surface Water/Soil Samples (GPS Coordinates) | Normalized IC50 Value (ng/mL) | Standard Deviation (ng/mL) |
| assay buffer | 12.5 | 0.4 |
| Lake Velencei at Agárd (47.190938, 18.584617) | 12.1 | 0.5 |
| Lake Velencei at Pákozd (47.213213, 18.577223) | 12.0 | 0.4 |
| Visegrád Trout Lake (47.774661, 18.986223) | 12.1 | 0.3 |
| feeding spring (47.773565, 18.985176) | 12.3 | 0.3 |
| Duna at Budapest (47.518549, 19.046216) | 11.9 | 0.6 |
| Balaton at Tihany (46.913958, 17.893470) | 12.0 | 0.4 |
| soil sample | 9.7 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takács, E.; Gémes, B.; Szendrei, F.; Keszei, C.; Barócsi, A.; Lenk, S.; Domján, L.; Mörtl, M.; Székács, A. Utilization of a Novel Immunofluorescence Instrument Prototype for the Determination of the Herbicide Glyphosate. Molecules 2022, 27, 6514. https://doi.org/10.3390/molecules27196514
Takács E, Gémes B, Szendrei F, Keszei C, Barócsi A, Lenk S, Domján L, Mörtl M, Székács A. Utilization of a Novel Immunofluorescence Instrument Prototype for the Determination of the Herbicide Glyphosate. Molecules. 2022; 27(19):6514. https://doi.org/10.3390/molecules27196514
Chicago/Turabian StyleTakács, Eszter, Borbála Gémes, Fanni Szendrei, Csaba Keszei, Attila Barócsi, Sándor Lenk, László Domján, Mária Mörtl, and András Székács. 2022. "Utilization of a Novel Immunofluorescence Instrument Prototype for the Determination of the Herbicide Glyphosate" Molecules 27, no. 19: 6514. https://doi.org/10.3390/molecules27196514
APA StyleTakács, E., Gémes, B., Szendrei, F., Keszei, C., Barócsi, A., Lenk, S., Domján, L., Mörtl, M., & Székács, A. (2022). Utilization of a Novel Immunofluorescence Instrument Prototype for the Determination of the Herbicide Glyphosate. Molecules, 27(19), 6514. https://doi.org/10.3390/molecules27196514