Photoluminescence Mechanism of Carbon Dots: Triggering Multiple Color Emissions through Controlling the Degree of Protonation
Abstract
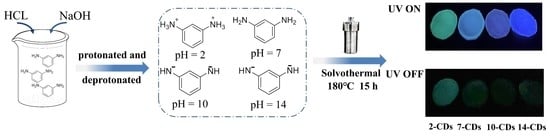
:1. Introduction
2. Results and Discussion
2.1. Formation and Structure
2.2. The PL Properties
2.3. The PL Mechanism of CDs
2.4. Application
2.4.1. The Ratiometric Fluorescent Sensor
2.4.2. Anti-Counterfeiting
3. Experimental Section
3.1. Materials
3.2. Characterizations
3.3. Preparation of CDs
3.4. Fabrication of CDs-PVA Film
3.5. Cu2+ Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhou, W.; Zheng, L.; Ning, Z.j.; Cheng, X.H.; Wang, F.; Xu, K.M.; Xu, R.; Liu, Z.Y.; Luo, M.; Hu, W.D.; et al. Silicon: Quantum dot photovoltage triodes. Nat. Commun. 2021, 12, 6696. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhou, W.J.; Ning, Z.j.; Wang, G.; Cheng, X.H.; Hu, W.D.; Zhou, W.; Liu, Z.D.; Yang, S.W.; Xu, K.M.; et al. Ambipolar Graphene–Quantum Dot Phototransistors with CMOS Compatibility. Adv. Opt. Mater. 2018, 6, 1800985. [Google Scholar] [CrossRef]
- Sinha, S.; Kim, P.H.; Switzer, C. 2′,5′-linked DNA is a template for polymerase-directed DNA synthesis. J. Am. Chem. Soc. 2004, 126, 40–41. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.X.; Zhang, N.; Yang, L.Y. Long-wavelength emissive solid-state carbon dots in nanoporous glass with excellent thermal stability. J. Colloid Interface Sci. 2021, 599, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.K.; Zhang, X.D.; Xia, L.Y.; Wang, H.Y.; Chen, Z.; Wu, F.G. One-step synthesis of ultrasmall and ultrabright organosilica nanodots with 100% photoluminescence quantum yield: Long-term lysosome imaging in living, fixed, and permeabilized cells. Nano Lett. 2018, 18, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Tang, B.J.; Wang, L.; Bao, H.; Lu, Y.H.; Guan, C.T.; Zhang, L.; Le, M.Y.; Liu, Z.; Wu, M.H. Machine-learning-driven synthesis of carbon dots with enhanced quantum yields. ACS Nano 2020, 14, 14761–14768. [Google Scholar] [CrossRef]
- Guo, J.Z.; Lu, Y.S.; Xie, A.Q.; Liang, Z.B.; Wang, C.F.; Yang, X.N.; Chen, S. Emissive carbon dots with high solid-state photoluminescence. Adv. Funct. Mater. 2022, 32, 2110393. [Google Scholar] [CrossRef]
- Xia, C.L.; Zhu, S.J.; Feng, T.L.; Yang, M.X.; Yang, B. Evolution and synthesis of carbon dots: From carbon dots to carbonized polymer dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Wei, G.; Zhang, W.J.; Wang, Z.Y.; Cheng, Y.X.; Dai, Z.H. Solid phase reaction method for preparation of carbon dots and multi-purpose applications. Sens. Actuators B 2016, 234, 15–20. [Google Scholar] [CrossRef]
- Qi, B.P.; Bao, L.; Zhang, Z.L.; Pang, D.W. Photoluminescent carbon nanodots: Preparation, photoluminescence mechanism and sensing. ACS Appl. Mater. Interfaces 2016, 42, 28372–28382. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Wen, Y.M.; Kang, X.H. Halogen-doped carbon dots: Synthesis, application, and prospects. Molecules 2022, 27, 4620. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gong, Z.J.; Tang, W.Y.; Row, K.H.; Qiu, H.D. Carbon dots in sample preparation and chromatographic separation: Recent advances and future prospects. TrAC Trends Anal. Chem. 2021, 134, 116135. [Google Scholar] [CrossRef]
- Gao, X.Q.; Wang, L.; Sun, C.; Zhou, N. Research on preparation methods of carbon nanomaterials based on self-assembly of carbon quantum dots. Molecules 2022, 27, 1690. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.J.; Yu, F.Y.; Lang, Z.L.; Nie, H.D.; Wang, Z.Z.; Shao, M.W.; Liu, Y.; Tan, H.Q.; Li, Y.G.; Kang, Z.H. Carbon dots/PtW6O24 composite as efficient and stable electrocatalyst for hydrogen oxidation reaction in PEMFCs. Chem. Eng. J. 2021, 426, 130709. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Y.Y.; Xu, Y.L.; Ran, G.X.; He, Y.M.; Song, Q.J. Aggregation-induced room-temperature phosphorescence obtained from water-dispersible carbon dot-based composite materials. ACS Appl. Mater. Interfaces 2021, 12, 10791–10800. [Google Scholar] [CrossRef]
- Nie, H.; Li, M.J.; Li, Q.S.; Liang, S.J.; Tan, Y.Y.; Sheng, L.; Shi, W.; Zhang, S.X.A. Continuously tunable full-color emission and their application in ratiometric pH ensing. Chem. Mater. 2014, 26, 3104–3112. [Google Scholar] [CrossRef]
- Shuang, E.; Mao, Q.X.; Wang, J.H.; Chen, X.W. Carbon dots with tunable dual emissions: From the mechanism to the specific imaging of endoplasmic reticulum polarity. Nanoscale 2020, 12, 6852–6860. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Wang, B.L.; Yu, X.W.; Li, J.Y.; Shang, J.; Yu, J.H. Carbon dots in porous materials: Host-guest synergy for enhanced performance. Angew. Chem. Int. Ed. 2020, 59, 19390. [Google Scholar] [CrossRef]
- Shamsipur, M.; Barati, A.; Taherpour, A.A.; Jamshidi, M. Resolving the multiple emission centers in carbon dots: From fluorophore molecular states to aromatic domain states and carbon-core states. J. Phys. Chem. Lett. 2018, 9, 4189–4198. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, H.F.; Luo, P.G.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Jang, M.H.; Ha, H.Y.D.; Kim, J.H.; Cho, Y.H.; Seo, T.S. Facile synthetic method for pristine graphene quantum dots and graphene oxide quantum dots: Origin of blue and green luminescence. Adv. Mater. 2013, 25, 3657–3662. [Google Scholar] [CrossRef] [PubMed]
- Mokkath, J.H.; Schwingenschlögl, U. Tuning the optical response in carbon doped boron nitride nanodots. J. Mater. Chem. C 2014, 2, 8322–8327. [Google Scholar] [CrossRef]
- Zhua, S.J.; Zhao, X.H.; Song, Y.B.; Lua, S.Y.; Yang, B. Beyond bottom-up carbon nanodots: Citric-acid derived organic molecules. Nano Today 2016, 11, 128–132. [Google Scholar] [CrossRef]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.G.; Cai, C.Z.; Lin, H.W. Red, green, and blue luminescence by carbon dots: Full-color emission tuning and multicolor cellular imaging. Angew. Chem. Int. Ed. 2015, 54, 5360–5363. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Song, X.Z.; Li, Y.C.; Li, X.H.; Zhang, Y.; Xie, W.J.; Fan, L.Z. Carbon dots: An innovative luminescent nanomaterial. Aggregate 2022, 3, e108. [Google Scholar]
- Choudhury, S.D.; Chethodil, J.M.; Gharat, P.M.; Praseetha, P.K.; Pal, H. pH-Elicited Luminescence Functionalities of Carbon Dots: Mechanistic Insights. J. Phys. Chem. Lett. 2017, 8, 1389–1395. [Google Scholar] [CrossRef]
- Wang, W.J.; Wu, J.; Xing, Y.X.; Wang, Z.H. Solvent-dependent red emissive carbon dots and their applications in sensing and solid-state luminescence. Sens. Actuators B 2022, 360, 131645. [Google Scholar] [CrossRef]
- Jiang, K.; Zhang, L.; Lu, J.F.; Xu, C.X.; Cai, C.Z.; Lin, H.W. Triple-mode emission of carbon dots: Applications for advanced anti-counterfeiting. Angew. Chem. Int. Ed. 2016, 55, 7231–7235. [Google Scholar] [CrossRef]
- Liu, M.L.; Chen, B.B.; Li, C.M.; Huang, C.Z. Carbon dots: Synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Lia, H.T.; Zhang, H.; Lia, K.J.; Zhang, J.L.; Suna, M.M.; Su, B.X. Catalytic graphitization of coke carbon by iron: Understanding the evolution of carbon Structure, morphology and lattice fringes. Fuel 2020, 279, 118531. [Google Scholar] [CrossRef]
- Wang, X.L.; Wang, S.Q.; Hao, C.; Zhao, Y.G.; Song, X.X. Quantifying orientation and curvature in HRTEM lattice fringe micrographs of naturally thermally altered coals: New insights from a structural evolution perspective. Fuel 2022, 309, 122180. [Google Scholar] [CrossRef]
- Peng, H.; Travas-Seidic, J. Simple aqueous solution route to luminescent carbogenic dots from carbohydrates. Chem. Mater. 2009, 21, 5563–5565. [Google Scholar] [CrossRef]
- Garrido, M.; Gualandi, L.; Noja, S.D.; Filippini, G.; Bosia, S.; Prato, M. Synthesis and applications of amino-functionalized carbon nanomaterials. Chem. Commun. 2020, 56, 12698–12716. [Google Scholar] [CrossRef] [PubMed]
- Arkin, K.; Zheng, Y.X.; Hao, J.W.; Zhang, S.Y.; Shang, Q.K. Polychromatic carbon dots prepared from m-Phenylenediamine and urea as multifunctional fluorescent Probes. ACS Appl. Nano Mater. 2021, 4, 8500–8510. [Google Scholar] [CrossRef]
- Ding, H.; Wei, J.S.; Xiong, H.M. Nitrogen and sulfur co-doped carbon dots with strong blue luminescence. Nanoscale 2014, 6, 13817–13823. [Google Scholar] [CrossRef] [PubMed]
- Andreazza-Vignolle, C.; Andreazza, P.; Zhao, D.X.; Wu, Y.H.; Wang, Q.L.; Zhang, T.; Jiang, K.M. Color-switchable, emission-enhanced fluorescence realized by engineering C-dot@C-dot nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 20700–20708. [Google Scholar]
- Vedamalai, M.; Periasamy, A.P.; Wang, C.W.; Tseng, Y.T.; Ho, L.C.; Shih, C.C.; Chang, H.T. Carbon nanodots prepared from o-phenylenediamine for sensing of Cu2+ ions in cells. Nanoscale 2014, 6, 13119–13125. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, H.; Liu, J.; Yao, J.; Wang, R.; Shi, W.; Lu, C. Photoluminescence Mechanism of Carbon Dots: Triggering Multiple Color Emissions through Controlling the Degree of Protonation. Molecules 2022, 27, 6517. https://doi.org/10.3390/molecules27196517
Yi H, Liu J, Yao J, Wang R, Shi W, Lu C. Photoluminescence Mechanism of Carbon Dots: Triggering Multiple Color Emissions through Controlling the Degree of Protonation. Molecules. 2022; 27(19):6517. https://doi.org/10.3390/molecules27196517
Chicago/Turabian StyleYi, Hao, Jing Liu, Jian Yao, Ruixing Wang, Wenying Shi, and Chao Lu. 2022. "Photoluminescence Mechanism of Carbon Dots: Triggering Multiple Color Emissions through Controlling the Degree of Protonation" Molecules 27, no. 19: 6517. https://doi.org/10.3390/molecules27196517
APA StyleYi, H., Liu, J., Yao, J., Wang, R., Shi, W., & Lu, C. (2022). Photoluminescence Mechanism of Carbon Dots: Triggering Multiple Color Emissions through Controlling the Degree of Protonation. Molecules, 27(19), 6517. https://doi.org/10.3390/molecules27196517