Morphology, Mechanical, and Water Barrier Properties of Carboxymethyl Rice Starch Films: Sodium Hydroxide Effect
Abstract
:1. Introduction
2. Results and Discussion
2.1. Degree of Substitution (DS) of CMSr Powder
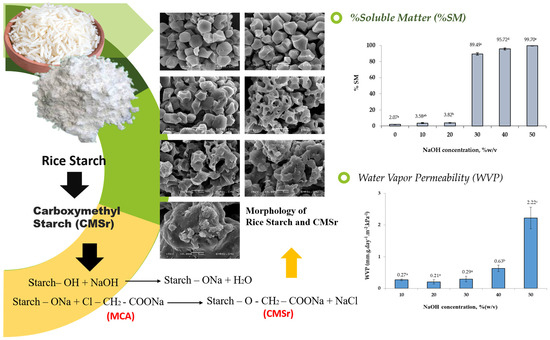
2.2. Scanning Electron Microscopy of CMSr Powders
2.3. FT-IR of Native Rice Starch Film and CMSr Films
2.4. SEM of Rice Starch Film and CMSr Films
2.5. X-ray Diffraction (XRD) of Native Rice Starch Film and CMSr Films
2.6. Differential Scanning Calorimetry (DSC) of Native Rice Starch Film and CMSr Films
2.7. Percentage of Soluble Matter (%SM) of Native Rice Starch Film and CMSr Films
2.8. Contact Angle of Native Rice Starch Film and CMSr Films
2.9. Water Vapor Permeability (WVP)
2.10. Tensile Strength (TS) and Elongation at Break (%E)
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Carboxymethyl Rice Starch (CMSr)
3.3. Degree of Substitution (DS)
3.4. Preparation of Native Rice Film and CMSr Films
3.5. Characterizations
3.5.1. Fourier Transform Infrared Spectroscopy (FT-IR)
3.5.2. Scanning Electron Microscopy (SEM)
3.5.3. X-ray Diffraction (XRD)
3.5.4. Differential Scanning Calorimetry (DSC)
3.5.5. Film Solubility
3.5.6. Dynamic Water Contact Angle
3.5.7. Film Thickness
3.5.8. Water Vapor Permeability
3.5.9. Tensile Strength
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pooresmaeil, M.; Namazi, H. Developments on carboxymethyl starch-based smart systems as promising drug carriers: A review. Carbohydr. Polym. 2021, 258, 117654. [Google Scholar] [CrossRef]
- Wilpiszewska, K.; Antosik, A.K.; Schmidt, B.; Janik, J.; Rokicka, J. Hydrophilic Films based on carboxymethylated derivatives of starch and cellulose. Polymers 2020, 12, 2447. [Google Scholar] [CrossRef]
- Spychaj, T.; Wilpiszewska, K.; Zdanowicz, M. Medium and high substituted carboxymethyl starch: Synthesis, characterization and application. Starch-Stärke 2013, 65, 22–33. [Google Scholar] [CrossRef]
- Stojanović, Ž.; Jeremić, K.; Jovanović, S.; Lechner, M.D. A comparison of some methods for the determination of the degree of substitution of carboxymethyl starch. Starch-Stärke 2005, 57, 79–83. [Google Scholar] [CrossRef]
- Li, S.F.; Mujyambere, J.M.V.; Liu, M. Synthesis of carboxymethyl starch with high degree of substitution by a modified dry process. Adv. Mat. Res. 2011, 233, 306–310. [Google Scholar] [CrossRef]
- Lawal, O.S.; Lechner, M.D.; Kulicke, W.M. The synthesis conditions, characterizations and thermal degradation studies of an etherified starch from an unconventional source. Polym. Degrad. Stab. 2008, 93, 1520–1528. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Li, X.; Xie, Q.; Tao, H.; Wang, W.; Chen, H.Q. Preparation and characterization of non-crystalline granular starch and corresponding carboxymethyl starch. Int. J. Biol. Macromol. 2017, 103, 656–662. [Google Scholar] [CrossRef]
- Zhang, B.; Gong, H.; Lü, S.; Ni, B.; Liu, M.; Gao, C.; Huang, Y.; Han, F. Synthesis and characterization of carboxymethyl potato starch and its application in reactive dye printing. Int. J. Biol. Macromol. 2012, 51, 668–674. [Google Scholar] [CrossRef]
- Kittipongpatana, O.; Sirithunyalug, J. Development of suspending agent from sodium carboxymethyl mungbean starches. Drug Dev. Ind. Pharm. 2006, 32, 809–820. [Google Scholar] [CrossRef]
- Kittipongpatana, O.S.; Chaitep, W.; Kittipongpatana, N.; Laenger, R.; Sriroth, K. Physicochemical and pharmaceutical properties of carboxymethyl rice starches modified from native starches with different amylose content. Cereal Chem. 2007, 84, 331–336. [Google Scholar] [CrossRef]
- Kittipongpatana, O.; Burapadaja, S.; Kittipongpatana, N. Development of pharmaceutical gel base containing sodium carboxymethyl mungbean starch. J. Nat. Sci. 2008, 7, 23. [Google Scholar]
- Suriyatem, R.; Auras, R.A.; Rachtanapun, P. Utilization of carboxymethyl cellulose from durian rind agricultural waste to improve physical properties and stability of rice starch-based film. J. Polym. Environ. 2019, 27, 286–298. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Luangkamin, S.; Tanprasert, K.; Suriyatem, R. Carboxymethyl cellulose film from durian rind. LWT-Food Sci. Technol. 2012, 48, 52–58. [Google Scholar] [CrossRef]
- Thanakkasaranee, S.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Jantrawut, P.; Ruksiriwanich, W.; Rose Sommano, S.; Punyodom, W. High Substitution synthesis of carboxymethyl chitosan for properties improvement of carboxymethyl chitosan films depending on particle sizes. Molecules 2021, 26, 6013. [Google Scholar] [CrossRef] [PubMed]
- Tongdeesoontorn, W.; Mauer, L.J.; Wongruong, S.; Sriburi, P.; Rachtanapun, P. Effect of carboxymethyl cellulose concentration on physical properties of biodegradable cassava starch-based films. Chem. Cent. J. 2011, 5, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rachtanapun, P.; Tongdeesoontorn, W. Effect of NaOH concentration on sorption isotherm of carboxymethyl rice starch films and prediction models. J. Sci. 2011, 38, 380–388. [Google Scholar]
- Domene-López, D.; García-Quesada, J.C.; Martin-Gullon, I.; Montalbán, M.G. Influence of starch composition and molecular weight on physicochemical properties of biodegradable films. Polymers 2019, 11, 1084. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and characterization of chitosan film incorporated with thinned young apple polyphenols as an active packaging material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Jeevahan, J.; Chandrasekaran, M. Influence of nanocellulose additive on the film properties of native rice starch-based edible films for food packaging. Recent Pat. Nanotechnol. 2019, 13, 222–233. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Huneault, M.A. Comparison of sorbitol and glycerol as plasticizers for thermoplastic starch in TPS/PLA blends. J. Appl. Polym. Sci. 2011, 119, 2439–2448. [Google Scholar] [CrossRef] [Green Version]
- Basiak, E.; Lenart, A.; Debeaufort, F. How glycerol and water contents affect the structural and functional properties of starch-based edible films. Polymers 2018, 10, 412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourtoom, T. Plasticizer effect on the properties of biodegradable blend film from rice starch-chitosan. Songklanakarin J. Sci. Technol. 2008, 30 (Suppl. 1), 149–165. [Google Scholar]
- Laohakunjit, N.; Noomhorm, A. Effect of plasticizers on mechanical and barrier properties of rice starch film. Starch-Stärke 2004, 56, 348–356. [Google Scholar] [CrossRef]
- Phan, T.D.; Debeaufort, F.; Luu, D.; Voilley, A. Functional properties of edible agar-based and starch-based films for food quality preservation. J. Agric. Food Chem. 2005, 53, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.-P.; Xia, W.-S. Physicochemical properties of edible and preservative films from chitosan/cassava starch/gelatin blend plasticized with glycerol. Food Technol. Biotechnol. 2008, 46, 262–269. [Google Scholar]
- Bourtoom, T.; Chinnan, M.S. Preparation and properties of rice starch–chitosan blend biodegradable film. LWT-Food Sci. Technol. 2008, 41, 1633–1641. [Google Scholar] [CrossRef]
- Suriyatem, R.; Auras, R.A.; Rachtanapun, P. Improvement of mechanical properties and thermal stability of biodegradable rice starch–based films blended with carboxymethyl chitosan. Ind. Crop. Prod. 2018, 122, 37–48. [Google Scholar] [CrossRef]
- Peressini, D.; Bravin, B.; Lapasin, R.; Rizzotti, C.; Sensidoni, A. Starch–methylcellulose based edible films: Rheological properties of film-forming dispersions. J. Food Eng. 2003, 59, 25–32. [Google Scholar] [CrossRef]
- Suriyatem, R.; Auras, R.A.; Rachtanapun, C.; Rachtanapun, P. Biodegradable rice starch/carboxymethyl chitosan films with added propolis extract for potential use as active food packaging. Polymers 2018, 10, 954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rachtanapun, P.; Simasatitkul, P.; Chaiwan, W.; Watthanaworasakun, Y. Effect of sodium hydroxide concentration on properties of carboxymethyl rice starch. Int. Food Res. J. 2012, 19, 923. [Google Scholar]
- Nattapulwat, N.; Purkkao, N.; Suwithayapan, O. Preparation and application of carboxymethyl yam (Dioscorea esculenta) starch. AAPS PharmSciTech 2009, 10, 193–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klunklin, W.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Insomphun, C.; Phongthai, S.; Jantrawut, P.; Sommano, S.R. Synthesis, characterization, and application of carboxymethyl cellulose from Asparagus stalk end. Polymers 2021, 13, 81. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Jantrawut, P.; Klunklin, W.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Insomphun, C.; Phongthai, S. Carboxymethyl bacterial cellulose from nata de coco: Effects of NaOH. Polymers 2021, 13, 348. [Google Scholar] [CrossRef] [PubMed]
- Rachtanapun, P.; Rattanapanone, N. Synthesis and characterization of carboxymethyl cellulose powder and films from Mimosa pigra. J. Appl. Polym. Sci. 2011, 122, 3218–3226. [Google Scholar] [CrossRef]
- Jaidee, A.; Rachtanapun, P.; Luangkamin, S. 1H-NMR analysis of degree of substitution in N,O-carboxymethyl chitosans from various chitosan sources and types. Adv. Mat. Res. 2012, 506, 158–161. [Google Scholar] [CrossRef]
- Barai, B.K.; Singhal, R.S.; Kulkarni, P.R. Optimization of a process for preparing carboxymethyl cellulose from water hyacinth (Eichornia crassipes). Carbohydr. Polym. 1997, 32, 229–231. [Google Scholar] [CrossRef]
- Pushpamalar, V.; Langford, S.J.; Ahmad, M.; Lim, Y.Y. Optimization of reaction conditions for preparing carboxymethyl cellulose from sago waste. Carbohydr. Polym. 2006, 64, 312–318. [Google Scholar] [CrossRef]
- Sulaiman, I.S.C.; Basri, M.; Masoumi, H.R.F.; Chee, W.J.; Ashari, S.E.; Ismail, M. Effects of temperature, time, and solvent ratio on the extraction of phenolic compounds and the anti-radical activity of Clinacanthus nutans Lindau leaves by response surface methodology. Chem. Cent. J. 2017, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Sangseethong, K.; Ketsilp, S.; Sriroth, K. The role of reaction parameters on the preparation and properties of carboxymethyl cassava starch. Starch-Stärke 2005, 57, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Bangar, S.P.; Purewal, S.S.; Trif, M.; Maqsood, S.; Kumar, M.; Manjunatha, V.; Rusu, A.V. Functionality and applicability of starch-based films: An eco-friendly approach. Foods 2021, 10, 2181. [Google Scholar] [CrossRef] [PubMed]
- Yanli, W.; Wenyuan, G.; Xia, L. Carboxymethyl chinese yam starch: Synthesis, characterization, and influence of reaction parameters. Carbohydr. Res. 2009, 344, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Thanakkasaranee, S.; Shin, H.; Ahn, K.; Sadeghi, K.; Lee, Y.; Tak, G.; Seo, J. Preparation and characterization of heat-resistant PET/bio-based polyester blends for hot-filled bottles. Polym. Test. 2020, 91, 106823. [Google Scholar] [CrossRef]
- Thanakkasaranee, S.; Sadeghi, K.; Lim, I.-J.; Seo, J. Effects of incorporating calcined corals as natural antimicrobial agent into active packaging system for milk storage. Mater. Sci. Eng. C 2020, 111, 110781. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, S.; Mahmoud, K.; Fatah, A.; Hassen, A. DSC, TGA and dielectric properties of carboxymethyl cellulose/polyvinyl alcohol blends. Phys. Rev. B Condens. Matter 2011, 406, 4068–4076. [Google Scholar] [CrossRef]
- Quadrado, R.F.; Fajardo, A.R. Microparticles based on carboxymethyl starch/chitosan polyelectrolyte complex as vehicles for drug delivery systems. Arab. J. Chem. 2020, 13, 2183–2194. [Google Scholar] [CrossRef]
- Drelich, J.; Chibowski, E.; Meng, D.D.; Terpilowski, K. Hydrophilic and superhydrophilic surfaces and materials. Soft Matter 2011, 7, 9804–9828. [Google Scholar] [CrossRef]
- Thanakkasaranee, S.; Kim, D.; Seo, J. Preparation and characterization of polypropylene/sodium propionate (PP/SP) composite films for bread packaging application. Packag. Technol. Sci. 2018, 31, 221–231. [Google Scholar] [CrossRef]
- Tavares, K.M.; de Campos, A.; Mitsuyuki, M.C.; Luchesi, B.R.; Marconcini, J.M. Corn and cassava starch with carboxymethyl cellulose films and its mechanical and hydrophobic properties. Carbohydr. Polym. 2019, 223, 115055. [Google Scholar] [CrossRef]
- Amaraweera, S.M.; Gunathilake, C.; Gunawardene, O.H.; Fernando, N.M.; Wanninayaka, D.B.; Dassanayake, R.S.; Rajapaksha, S.M.; Manamperi, A.; Fernando, C.A.; Kulatunga, A.K. Development of starch-based materials using current modification techniques and their applications: A Review. Molecules 2021, 26, 6880. [Google Scholar] [CrossRef] [PubMed]
- Thanakkasaranee, S.; Kim, D.; Seo, J. Preparation and characterization of poly (ether-block-amide)/polyethylene glycol composite films with temperature-dependent permeation. Polymers 2018, 10, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanakkasaranee, S.; Pradittham, A.; Atong, D.; Pechyen, C. Effect of nano-silica loading on barrier and mechanical properties of food packaging based LLDPE film. Adv. Mat. Res. 2012, 488, 919–922. [Google Scholar] [CrossRef]
- Nawaz, H.; Waheed, R.; Nawaz, M.; Shahwar, D. Physical and chemical modifications in starch structure and reactivity. In Chemical Properties of Starch; IntechOpen: London, UK, 2020; pp. 13–57. [Google Scholar]
- Rachtanapun, P.; Kumthai, S.; Mulkarat, N.; Pintajam, N.; Suriyatem, R. Value added of mulberry paper waste by carboxymethylation for preparation a packaging film. IOP Conf. Ser. Mater. Sci. Eng. 2015, 87, 012081. [Google Scholar] [CrossRef] [Green Version]
- Chaiwong, N.; Leelapornpisid, P.; Jantanasakulwong, K.; Rachtanapun, P.; Seesuriyachan, P.; Sakdatorn, V.; Leksawasdi, N.; Phimolsiripol, Y. Antioxidant and moisturizing properties of carboxymethyl chitosan with different molecular weights. Polymers 2020, 12, 1445. [Google Scholar] [CrossRef] [PubMed]











| Samples | Time (s) | |||||
|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | 50 | |
| Rice starch |  |  |  |  |  |  |
| 93.9° | 91.3° | 91.9° | 92.3° | 91.5° | 90.9° | |
| 10% NaOH |  |  |  |  |  |  |
| 92.4° | 91.0° | 91.5° | 91.3° | 90.8° | 90.6° | |
| 20% NaOH |  |  |  |  |  |  |
| 91.6° | 90.4° | 90.4° | 90.8° | 89.7° | 89.5° | |
| 30% NaOH |  |  |  |  |  |  |
| 79.8° | 76.4° | 74.7° | 72.3° | 71.1° | 68.7° | |
| 40% NaOH |  |  |  |  |  |  |
| 76.0° | 73.3° | 71.1° | 70.4° | 69.7° | 68.1° | |
| 50% NaOH |  |  |  |  |  |  |
| 72.5° | 69.8° | 68.2° | 66.6° | 65.6° | 64.3° | |
| Type of Film | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| 0% NaOH-CMSr | 10.75 ± 2.25 a | 7.56 ± 1.72 a |
| 10% NaOH-CMSr | 9.87 ± 1.42 a | 11.36 ± 2.18 b |
| 20% NaOH-CMSr | 4.39 ± 1.86 b | 22.98 ± 2.73 c |
| 30% NaOH-CMSr | 4.46 ± 1.73 b | 29.64 ± 3.08 d |
| 40% NaOH-CMSr | 2.75 ± 1.28 c | 53.03 ± 4.49 e |
| 50% NaOH-CMSr | 2.86 ± 1.02 c | 25.08 ± 1.16 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rachtanapun, P.; Thanakkasaranee, S.; Auras, R.A.; Chaiwong, N.; Jantanasakulwong, K.; Jantrawut, P.; Phimolsiripol, Y.; Seesuriyachan, P.; Leksawasdi, N.; Chaiyaso, T.; et al. Morphology, Mechanical, and Water Barrier Properties of Carboxymethyl Rice Starch Films: Sodium Hydroxide Effect. Molecules 2022, 27, 331. https://doi.org/10.3390/molecules27020331
Rachtanapun P, Thanakkasaranee S, Auras RA, Chaiwong N, Jantanasakulwong K, Jantrawut P, Phimolsiripol Y, Seesuriyachan P, Leksawasdi N, Chaiyaso T, et al. Morphology, Mechanical, and Water Barrier Properties of Carboxymethyl Rice Starch Films: Sodium Hydroxide Effect. Molecules. 2022; 27(2):331. https://doi.org/10.3390/molecules27020331
Chicago/Turabian StyleRachtanapun, Pornchai, Sarinthip Thanakkasaranee, Rafael A. Auras, Nareekan Chaiwong, Kittisak Jantanasakulwong, Pensak Jantrawut, Yuthana Phimolsiripol, Phisit Seesuriyachan, Noppol Leksawasdi, Thanongsak Chaiyaso, and et al. 2022. "Morphology, Mechanical, and Water Barrier Properties of Carboxymethyl Rice Starch Films: Sodium Hydroxide Effect" Molecules 27, no. 2: 331. https://doi.org/10.3390/molecules27020331
APA StyleRachtanapun, P., Thanakkasaranee, S., Auras, R. A., Chaiwong, N., Jantanasakulwong, K., Jantrawut, P., Phimolsiripol, Y., Seesuriyachan, P., Leksawasdi, N., Chaiyaso, T., Somman, S. R., Ruksiriwanich, W., Klunklin, W., Reungsang, A., & Ngo, T. M. P. (2022). Morphology, Mechanical, and Water Barrier Properties of Carboxymethyl Rice Starch Films: Sodium Hydroxide Effect. Molecules, 27(2), 331. https://doi.org/10.3390/molecules27020331