Volatile Organic Compound Fragmentation in the Afterglow of Pulsed Glow Discharge in Ambient Air
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Effect of Cathode Geometry
2.2. The Comparison of the Performance for the Argon–Air Mixture and Air
2.3. Specific Fragmentation of Alcohols
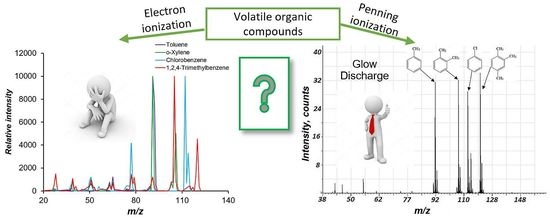
2.4. The Comparison of Penning Ionization in Glow Discharge and Electron Ionization
2.5. Limits of Detection
3. Materials and Methods
3.1. Instrumentation
3.2. Samples and Sample Introduction
3.3. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Linstrom, P.J.; Mallard, W.G. NIST Standard Reference Database Number 69; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2021; p. 20899.
- Yuan, B.; Koss, A.R.; Warneke, C.; Coggon, M.; Sekimoto, K.; de Gouw, J.A. Proton-Transfer-Reaction Mass Spectrometry: Applications in Atmospheric Sciences. Chem. Rev. 2017, 117, 13187–13229. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Španěl, P. Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis. Mass Spectrom. Rev. 2005, 24, 661–700. [Google Scholar] [CrossRef] [PubMed]
- Nunome, Y.; Kodama, K.; Ueki, Y.; Yoshiie, R.; Naruse, I.; Wagatsuma, K. Development of soft ionization using direct current pulse glow discharge plasma source in mass spectrometry for volatile organic compounds analysis. Spectrochim. Acta Part B At. Spectrosc. 2018, 139, 44–49. [Google Scholar] [CrossRef]
- Lindinger, W.; Hansel, A.; Jordan, A. On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR-MS) medical applications, food control and environmental research. Int. J. Mass Spectrom. Ion Processes 1998, 173, 191–241. [Google Scholar] [CrossRef]
- Olivenza-León, D.; Mayhew, C.A.; González-Méndez, R. Proton transfer reaction mass spectrometry investigations of phthalate esters via direct headspace sampling. Int. J. Mass Spectrom. 2021, 461, 116497. [Google Scholar] [CrossRef]
- Hansel, A.; Jordan, A.; Holzinger, R.; Prazeller, P.; Vogel, W.; Lindinger, W. Proton transfer reaction mass spectrometry: On-line trace gas analysis at the ppb level. Int. J. Mass Spectrom. Ion Processes 1995, 149–150, 609–619. [Google Scholar] [CrossRef]
- De Gouw, J.A.; Goldan, P.D.; Warneke, C.; Kuster, W.C.; Roberts, J.M.; Marchewka, M.; Bertman, S.B.; Pszenny, A.A.P.; Keene, W.C. Validation of proton transfer reaction-mass spectrometry (PTR-MS) measurements of gas-phase organic compounds in the atmosphere during the New England Air Quality Study (NEAQS) in 2002. J. Geophys. Res. Atmos. 2003, 108, D21. [Google Scholar] [CrossRef]
- De Gouw, J.; Warneke, C. Measurements of volatile organic compounds in the earth’s atmosphere using proton-transfer-reaction mass spectrometry. Mass Spectrom. Rev. 2007, 26, 223–257. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Haidacher, S.; Hanel, G.; Hartungen, E.; Herbig, J.; Märk, L.; Schottkowsky, R.; Seehauser, H.; Sulzer, P.; Märk, T.D. An online ultra-high sensitivity Proton-transfer-reaction mass-spectrometer combined with switchable reagent ion capability (PTR+SRI−MS). Int. J. Mass Spectrom. 2009, 286, 32–38. [Google Scholar] [CrossRef]
- Sulzer, P.; Edtbauer, A.; Hartungen, E.; Jürschik, S.; Jordan, A.; Hanel, G.; Feil, S.; Jaksch, S.; Märk, L.; Märk, T.D. From conventional proton-transfer-reaction mass spectrometry (PTR-MS) to universal trace gas analysis. Int. J. Mass Spectrom. 2012, 321–322, 66–70. [Google Scholar] [CrossRef]
- Müller, M.; Piel, F.; Gutmann, R.; Sulzer, P.; Hartungen, E.; Wisthaler, A. A novel method for producing NH4+ reagent ions in the hollow cathode glow discharge ion source of PTR-MS instruments. Int. J. Mass Spectrom. 2020, 447, 116254. [Google Scholar] [CrossRef]
- Salazar Gómez, J.I.; Klucken, C.; Sojka, M.; Masliuk, L.; Lunkenbein, T.; Schlögl, R.; Ruland, H. Elucidation of artefacts in proton transfer reaction time-of-flight mass spectrometers. J. Mass Spectrom. 2019, 54, 987–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehnert, A.S.; Behrendt, T.; Ruecker, A.; Pohnert, G.; Trumbore, S.E. SIFT-MS optimization for atmospheric trace gas measurements at varying humidity. Atmos. Meas. Tech. 2020, 13, 3507–3520. [Google Scholar] [CrossRef]
- Langford, V.S.; Padayachee, D.; McEwan, M.J.; Barringer, S.A. Comprehensive odorant analysis for on-line applications using selected ion flow tube mass spectrometry (SIFT-MS). Flavour Fragr. J. 2019, 34, 393–410. [Google Scholar] [CrossRef]
- Hastie, C.; Thompson, A.; Perkins, M.; Langford, V.S.; Eddleston, M.; Homer, N.Z.M. Selected Ion Flow Tube-Mass Spectrometry (SIFT-MS) as an Alternative to Gas Chromatography/Mass Spectrometry (GC/MS) for the Analysis of Cyclohexanone and Cyclohexanol in Plasma. ACS Omega 2021, 6, 32818–32822. [Google Scholar] [CrossRef] [PubMed]
- Gubal, A.; Chuchina, V.; Sorokina, A.; Solovyev, N.; Ganeev, A. Mass spectrometry-based techniques for direct quantification of high ionization energy elements in solid materials—challenges and perspectives. Mass Spectrom. Rev. 2021, 40, 359–380. [Google Scholar] [CrossRef]
- Gubal, A.; Chuchina, V.; Lyalkin, Y.; Ivanenko, N.; Solovyev, N.; Stroganov, A.; Ganeev, A. New Possibilities for the Determination of Volatile Organic Compounds by Their Molecular Ions in Air Using µs-Pulsed GD TOFMS. At. Spectrosc. 2021, 42, 120–127. [Google Scholar] [CrossRef]
- Nunome, Y.; Park, H.; Kodama, K.; Ueki, Y.; Yoshiie, R.; Lee, S.C.; Kitagawa, K.; Wagatsuma, K.; Naruse, I. Use of Soft Plasma Ionization Source at Evacuated Air Atmospheres in Time-of-Flight Mass Spectrometry to Suppress Fragmentation of Volatile Organic Compounds. Spectrosc. Lett. 2015, 48, 436–440. [Google Scholar] [CrossRef]
- Nunome, Y.; Kodama, K.; Ueki, Y.; Yoshiie, R.; Naruse, I.; Wagatsuma, K. Direct analysis of saturated hydrocarbons using glow discharge plasma ionization source for mass spectrometry. Talanta 2019, 204, 310–319. [Google Scholar] [CrossRef]
- Fandino, J.; Bouza, M.; Pisonero, J.; Blanco, D.; Sanz-Medel, A.; Bordel, N. A novel gas sampling introduction interface for fast analysis of volatile organic compounds using radiofrequency pulsed glow discharge time of flight mass spectrometry. Anal. Chim. Acta 2018, 1038, 59–66. [Google Scholar] [CrossRef]
- Bouza, M.; Fandino, J.; Bordel, N.; Pereiro, R.; Sanz-Medel, A. Volatile organic compound analysis by pulsed glow discharge time of flight mass spectrometry as a structural elucidation tool. J. Mass Spectrom. 2017, 52, 561–570. [Google Scholar] [CrossRef]
- Fandino, J.; Orejas, J.; Chauvet, L.; Blanco, D.; Guillot, P.; Pisonero, J.; Bordel, N. Evaluation of a modified halo flowing atmospheric pressure afterglow ion source for the analysis of directly injected volatile organic compounds. J. Anal. At. Spectrom. 2020, 35, 2002–2010. [Google Scholar] [CrossRef]
- Gubal, A.; Chuchina, V.; Ivanenko, N.; Qian, R.; Solovyev, N.; Ganeev, A. Microsecond pulsed glow discharge in copper hollow cathode reveals a new approach to ionization and determination of volatile organic compounds. Spectrochim. Acta Part B At. Spectrosc. 2020, 173, 105986. [Google Scholar] [CrossRef]
- Bodnar, V.; Ganeev, A.; Gubal, A.; Solovyev, N.; Glumov, O.; Yakobson, V.; Murin, I. Pulsed glow discharge enables direct mass spectrometric measurement of fluorine in crystal materials—Fluorine quantification and depth profiling in fluorine doped potassium titanyl phosphate. Spectrochim. Acta B 2018, in press. [CrossRef]
- Ganeev, A.A.; Gubal, A.R.; Lukyanov, G.N.; Arseniev, A.I.; Barchuk, A.A.; Jahatspanian, I.E.; Gorbunov, I.S.; Rassadina, A.A.; Nemets, V.M.; Nefedov, A.O.; et al. Analysis of exhaled air for early-stage diagnosis of lung cancer: Opportunities and challenges. Russ. Chem. Rev. 2018, 87, 904–921. [Google Scholar] [CrossRef]
- Moskalev, B. Discharge with a Hollow Cathode; Energoizdat: Moscow, Russia, 1969; p. 89. [Google Scholar]
- Gubal, A.; Ganeev, A.; Hoffmann, V.; Voronov, M.; Brackmann, V.; Oswald, S. Combined hollow cathode vs. Grimm cell: Semiconductive and nonconductive samples. J. Anal. At. Spectrom. 2017, 32, 354–366. [Google Scholar] [CrossRef]
- Barber, S.; Blake, R.S.; White, I.R.; Monks, P.S.; Reich, F.; Mullock, S.; Ellis, A.M. Increased Sensitivity in Proton Transfer Reaction Mass Spectrometry by Incorporation of a Radio Frequency Ion Funnel. Anal. Chem. 2012, 84, 5387–5391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, R.; Beig, G.; Anand, V.; Kalbande, R.; Maji, S. Tracer-based characterization of source variations of ambient isoprene mixing ratios in a hillocky megacity, India, influenced by the local meteorology. Environ. Res. 2022, 205, 112465. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Mikoviny, T.; Feil, S.; Haidacher, S.; Hanel, G.; Hartungen, E.; Jordan, A.; Märk, L.; Mutschlechner, P.; Schottkowsky, R.; et al. A compact PTR-ToF-MS instrument for airborne measurements of volatile organic compounds at high spatiotemporal resolution. Atmos. Meas. Tech. 2014, 7, 3763–3772. [Google Scholar] [CrossRef]
- Murray Kermit, K.; Boyd Robert, K.; Eberlin Marcos, N.; Langley, G.J.; Li, L.; Naito, Y. Definitions of terms relating to mass spectrometry (IUPAC Recommendations 2013). pac 2013, 85, 1515. [Google Scholar]
- Marcus, R.K.; Broekaert, J.A.C. Glow Discharge Plasmas in Analytical Spectroscopy; John Wiley & Sons, Ltd.: Chichester, UK, 2003; p. 498. [Google Scholar]





| VOC | Molecular Weight, Da | m/z | PGD-TOFMS, Relative Intensities of Ions, % | EI, Relative Intensities of Ions, % [1] |
|---|---|---|---|---|
| Alkanes | ||||
| n-Heptane | 100 | 100 | 67 | 15 |
| 71 | 100 | 45 | ||
| 70 | 35 | 18 | ||
| 57 | 44 | 47 | ||
| 56 | 35 | 27 | ||
| 43 | 13 | 100 | ||
| 42 | 8 | 25 | ||
| n-Octane | 114 | 114 | 21 | 6 |
| 85 | 100 | 26 | ||
| 71 | 62 | 20 | ||
| 57 | 33 | 34 | ||
| 43 | 27 | 100 | ||
| 41 | 5 | 44 | ||
| 29 | 3 | 27 | ||
| Arenes | ||||
| Ethylbenzene | 106 | 106 | 100 | 28 |
| 91 | 100 | 100 | ||
| 77 | - | 10 | ||
| 65 | - | 11 | ||
| 51 | - | 11 | ||
| 39 | - | 7 | ||
| Alcohols | ||||
| Propanol-1 | 60 | 60 | 2.5 | 7 |
| 88 | 68 (MN2+) | - | ||
| 59 | 48 | 12 | ||
| 58 | 100 | 0 | ||
| 31 | 34 | 100 | ||
| Carboxylic acids | ||||
| Propionic acid | 74 | 74 | 100 | 100 |
| 57 | 21 | 46 | ||
| 45 | 5 | 90 | ||
| 43 | 37 | 6 | ||
| 29 | 4 | 85 | ||
| 28 | 15 | 94 | ||
| 27 | - | 63 | ||
| 93 | 29 (M + H3O+) | - | ||
| 104 | 14 (MNO+) | - | ||
| VOC | LODs, ppb |
|---|---|
| Toluene | 2.0 |
| Chlorobenzene | 3.0 |
| p-Xylene | 0.5 |
| 1,2,4-Trimethylbenzene | 5.0 |
| o-Xylene | 0.5 |
| Butanol-1 | 6.0 |
| Propanol-1 | 4.0 |
| Ethanol | 3.0 |
| n-Heptane | 2.0 |
| Methyl acetate | 1.0 |
| Propyl acetate | 1.5 |
| Butyl acetate | 4.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kravtsov, D.; Gubal, A.; Chuchina, V.; Ivanenko, N.; Solovyev, N.; Stroganov, A.; Jin, H.; Ganeev, A. Volatile Organic Compound Fragmentation in the Afterglow of Pulsed Glow Discharge in Ambient Air. Molecules 2022, 27, 6864. https://doi.org/10.3390/molecules27206864
Kravtsov D, Gubal A, Chuchina V, Ivanenko N, Solovyev N, Stroganov A, Jin H, Ganeev A. Volatile Organic Compound Fragmentation in the Afterglow of Pulsed Glow Discharge in Ambient Air. Molecules. 2022; 27(20):6864. https://doi.org/10.3390/molecules27206864
Chicago/Turabian StyleKravtsov, Denis, Anna Gubal, Victoria Chuchina, Natalya Ivanenko, Nikolay Solovyev, Alexander Stroganov, Han Jin, and Alexander Ganeev. 2022. "Volatile Organic Compound Fragmentation in the Afterglow of Pulsed Glow Discharge in Ambient Air" Molecules 27, no. 20: 6864. https://doi.org/10.3390/molecules27206864
APA StyleKravtsov, D., Gubal, A., Chuchina, V., Ivanenko, N., Solovyev, N., Stroganov, A., Jin, H., & Ganeev, A. (2022). Volatile Organic Compound Fragmentation in the Afterglow of Pulsed Glow Discharge in Ambient Air. Molecules, 27(20), 6864. https://doi.org/10.3390/molecules27206864