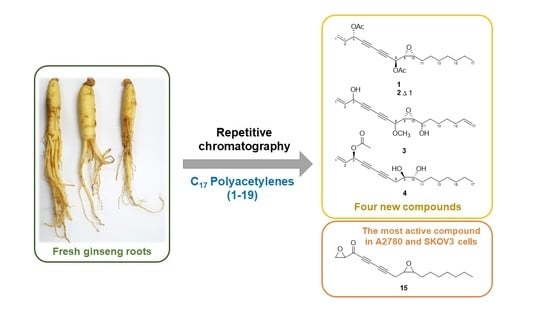
Cytotoxic Properties of C17 Polyacetylenes from the Fresh Roots of Panax ginseng on Human Epithelial Ovarian Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Structure Elucidation of Isolated Compounds
2.2. The Cytotoxicity of C17 Polyacetylenes against Human Ovarian Cancer Cells
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedure
4.2. Plant Materials
4.3. Extraction and Isolation of Polyacetylenes from P. ginseng
4.3.1. Ginsenoyne O (1)
4.3.2. Ginsenoyne P (2)
4.3.3. Ginsenoyne Q (3)
4.3.4. 3-Acetyl Panaxytriol (4)
4.4. Computational Chemistry
4.5. Deacetylation of Compound 4
4.6. Cell Culture and MTT Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clark, M.A.; Springmann, M.; Hill, J.; Tilman, D. Multiple health and environmental impacts of foods. Proc. Natl. Acad. Sci. USA 2019, 116, 23357–23362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, L.M.; Pinsky, P.F.; Trabert, B. General population screening for ovarian cancer. Lancet 2021, 397, 2128–2130. [Google Scholar] [CrossRef]
- Kaku, T.; Ogawa, S.; Kawano, Y.; Ohishi, Y.; Kobayashi, H.; Hirakawa, T.; Nakano, H. Histological classification of ovarian cancer. Med. Electron Microsc. 2003, 3, 9–17. [Google Scholar] [CrossRef]
- Kim, A.; Ueda, Y.; Naka, T.; Enomoto, T. Therapeutic strategies in epithelial ovarian cancer. J. Exp. Clin. Cancer Res. 2012, 31, 14. [Google Scholar] [CrossRef] [Green Version]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Helleman, J.; Smid, M.; Jansen, M.P.; van der Burg, M.E.; Berns, E.M. Pathway analysis of gene lists associated with platinum-based chemotherapy resistance in ovarian cancer: The big picture. Gynecol. Oncol. 2010, 117, 170–176. [Google Scholar] [CrossRef]
- Mann, J. Natural products in cancer chemotherapy: Past, present and future. Nat. Rev. Cancer 2002, 2, 143–148. [Google Scholar] [CrossRef]
- Ru, W.; Wang, D.; Xu, Y.; He, X.; Sun, Y.E.; Qian, L.; Zhou, X.; Qin, Y. Chemical constituents and bioactivities of Panax ginseng (CA Mey.). Drug Discov. Ther. 2015, 9, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.B.; Tian, Q.; Zhang, J.F.; Xiang, Y. Antitumor effects and molecular mechanisms of action of natural products in ovarian cancer. Oncol. Lett. 2020, 20, 141. [Google Scholar] [CrossRef]
- Christensen, L.P. Bioactive C17 and C18 acetylenic oxylipins from terrestrial plants as potential lead compounds for anticancer drug development. Molecules 2020, 25, 2568. [Google Scholar] [CrossRef]
- Matsumori, N.; Kaneno, D.; Murata, M.; Nakamura, H.; Tachibana, K. Stereochemical determination of acyclic structures based on carbon-proton spin-coupling constants. A method of configuration analysis for natural products. J. Org. Chem. 1999, 64, 866–876. [Google Scholar] [CrossRef]
- Yang, M.C.; Kwon, H.C.; Kim, Y.J.; Lee, K.R.; Yang, H.O. Oploxynes A and B, polyacetylenes from the stems of Oplopanax elatus. J. Nat. Prod. 2010, 73, 801–805. [Google Scholar] [CrossRef]
- Yadav, J.; Boyapelly, K.; Alugubelli, S.R.; Pabbaraja, S.; Vangala, J.R.; Kalivendi, S.V. Stereoselective total synthesis of (+)-oploxyne A, (−)-oploxyne B, and their C-10 epimers and structure revision of natural oploxyne B. J. Org. Chem. 2011, 76, 2568–2576. [Google Scholar] [CrossRef]
- Giorgio, E.; Viglione, R.G.; Zanasi, R.; Rosini, C. Ab initio calculation of optical rotatory dispersion (ORD) curves: A simple and reliable approach to the assignment of the molecular absolute configuration. J. Am. Chem. Soc. 2004, 126, 12968–12976. [Google Scholar] [CrossRef]
- Kim, S.I.; Lee, Y.H.; Kang, K.S. 10-Acetyl panaxytriol, a new cytotoxic polyacetylene from Panax ginseng. Yakhak Hoeji 1989, 33, 118–123. [Google Scholar]
- Satoh, M.; Ishii, M.; Watanabe, M.; Isobe, K.; Uchiyama, T.; Fujimoto, Y. Absolute structure of panaxytriol. Chem. Pharm. Bull. 2002, 50, 126–128. [Google Scholar] [CrossRef] [Green Version]
- Hirakura, K.; Morita, M.; Nakajima, K.; Ikeya, Y.; Mitsuhashi, H. Polyacetylenes from the roots of Panax ginseng. Phytochemistry 1991, 30, 3327–3333. [Google Scholar] [CrossRef]
- Satoh, M.; Watanabe, M.; Kawahata, M.; Mohri, K.; Yoshida, Y.; Isobe, K.; Fujimoto, Y. Synthesis of panax acetylenes: Chiral syntheses of acetylpanaxydol, PQ-3 and panaxydiol. Chem. Pharm. Bull. 2004, 52, 418–421. [Google Scholar] [CrossRef] [Green Version]
- Hirakura, K.; Morita, M.; Nakajima, K.; Ikeya, Y.; Mitsuhashi, H. Three acetylated polyacetylenes from the roots of Panax ginseng. Phytochemistry 1991, 30, 4053–4055. [Google Scholar] [CrossRef]
- Xu, G.H.; Choo, S.J.; Ryoo, I.J.; Kim, Y.H.; Paek, K.Y.; Yoo, I.D. Polyacetylenes from the tissue cultured adventitious roots of Panax ginseng CA Meyer. Nat. Prod. Sci. 2008, 14, 177–181. [Google Scholar]
- Shim, S.Y.; Sung, S.; Lee, M. Ginsenoyne C, a polyacetylene isolated from Panax Ginseng inhibit inflammatory mediators via regulating extracellular regulated kinases signaling. Pharmacogn. Mag. 2018, 14, 358–362. [Google Scholar]
- Knispel, N.; Ostrozhenkova, E.; Schramek, N.; Huber, C.; Peña-Rodríguez, L.M.; Bonfill, M.; Palazón, J.; Wischmann, G.; Cusidó, R.M.; Eisenreich, W. Biosynthesis of panaxynol and panaxydol in Panax ginseng. Molecules 2013, 18, 7686–7698. [Google Scholar] [CrossRef]
- Bohlmann, F.; Arndt, C.; Bornowski, H.; Kleine, K. Polyacetylene compounds. XXXI. Polyynes from the family of umbellifers. Chem. Ber. 1961, 94, 958–967. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Hongcheng, W.; Kirisawa, M.; Satoh, M.; Takeuchi, N. Acetylenes from Panax quinquefolium. Phytochemistry 1992, 31, 3499–3501. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, K.; Rho, M.C.; Chung, M.Y.; Kim, Y.H.; Lee, S.; Lee, H.S.; Kim, Y.K. New polyacetylenes, DGAT inhibitors from the roots of Panax ginseng. Planta Med. 2004, 70, 197–200. [Google Scholar] [PubMed]
- Ning, J.; Di, Y.T.; Li, S.F.; Geng, Z.L.; He, H.P.; Wang, Y.H.; Wang, Y.Y.; Li, Y.; Li, S.L.; Hao, X.J. Polyynes from Toona ciliata var. ciliata and related cytotoxic activity. Helv. Chim. Acta 2011, 94, 376–381. [Google Scholar] [CrossRef]
- Christensen, L.P.; Martin, J.; Ulla, K. Simultaneous determination of ginsenosides and polyacetylenes in American ginseng root (Panax quinquefolium L.) by high-performance liquid chromatography. J. Agric. Food Chem. 2006, 54, 8995–9003. [Google Scholar] [CrossRef]
- Yeo, C.R.; Yong, J.J.; Popovich, D.G. Isolation and characterization of bioactive polyacetylenes Panax ginseng Meyer roots. J. Pharm. Biomed. Anal. 2017, 139, 148–155. [Google Scholar] [CrossRef]
- Rojas, V.; Hirshfield, K.M.; Ganesan, S. Rodriguez-Rodriguez, L. Molecular characterization of epithelial ovarian cancer: Implications for diagnosis and treatment. Int. J. Mol. Sci. 2016, 17, 2113. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.C.; Seo, D.S.; Choi, S.U.; Park, Y.H.; Lee, K.R. Polyacetylenes from the roots of cultivated-wild ginseng and their cytotoxicity in vitro. Arch. Pharm. Res. 2008, 31, 154–159. [Google Scholar] [CrossRef]
- Mao, J.; Zhong, J.; Wang, B.; Jin, J.; Li, S.; Gao, Z.; Yang, H.; Bian, Q. Synthesis of panaxytriol and its stereoisomers as potential antitumor drugs. Tetrahedron Asymmetry 2016, 27, 330–337. [Google Scholar] [CrossRef]
- Mao, J.; Li, S.; Zhong, J.; Wang, B.; Jin, J.; Gao, Z.; Yang, H.; Bian, Q. Total synthesis of panaxydol and its stereoisomers as potential anticancer agents. Tetrahedron Asymmetry 2016, 27, 69–77. [Google Scholar] [CrossRef]
- Pollo, L.A.; Bosi, C.F.; Leite, A.S.; Rigotto, C.; Kratz, J.; Simões, C.M.; Fonseca, D.E.; Coimbra, D.; Caramori, G.; Nepel, A. Polyacetylenes from the leaves of Vernonia scorpioides (Asteraceae) and their antiproliferative and antiherpetic activities. Phytochemistry 2013, 95, 375–383. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, H.Y.; Kim, H.M.; Choi, J.H.; Jang, D.S. A novel cytotoxic steroidal saponin from the roots of Asparagus cochinchinensis. Plants 2021, 10, 2067. [Google Scholar] [CrossRef]
- Seo, Y.J.; Jeong, M.; Lee, K.T.; Jang, D.S.; Choi, J.H. Isocyperol, isolated from the rhizomes of Cyperus rotundus, inhibits LPS-induced inflammatory responses via suppression of the NF-κB and STAT3 pathways and ROS stress in LPS-stimulated RAW 264.7 cells. Int. Immunopharmacol. 2016, 38, 61–69. [Google Scholar] [CrossRef]

 ) correlations among compounds 1–4.
) correlations among compounds 1–4.
 ) between compounds 1 and 2 (A,B). The 3D structures of 1 and 2 were modeled by Spartan′ 18.
) between compounds 1 and 2 (A,B). The 3D structures of 1 and 2 were modeled by Spartan′ 18.
 ) between compounds 1 and 2 (A,B). The 3D structures of 1 and 2 were modeled by Spartan′ 18.
) between compounds 1 and 2 (A,B). The 3D structures of 1 and 2 were modeled by Spartan′ 18.


| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δH (multi J in Hz) a | δC b | δH (multi J in Hz) a | δCc | δH (multi J in Hz) a | δCd | |
| 1 | 1.01 (7.5) | 9.5 | 5.33 d (10.0) 5.52 d (16.5) | 120.1 | 0.99 t (7.5) | 9.5 |
| 2 | 1.80 qd (7.5, 6.5) | 28.0 | 5.83 ddd (15.5, 10.0, 6.5) | 131.8 | 1.79 d (6.0) | 30.8 |
| 3 | 5.33 td (6.5, 0.5) | 65.3 | 5.88 td (6.5, 1.0) | 64.3 | 4.36 dd (12.0, 6.0) | 64.3 |
| 4 | 74.4 | 74.9 | 81.2 | |||
| 5 | 70.8 | 70.4 | 72.4 | |||
| 6 | 69.3 | 70.5 | 69.2 | |||
| 7 | 77.7 | 75.2 | 68.8 | |||
| 8 | 5.23 dd (7.5, 0.5) | 61.9 | 5.22 dd (7.5, 0.5) | 61.7 | 4.01 d (7.0) | 70.9 |
| 9 | 3.21 dd (7.5, 4.0) | 56.6 | 3.19 dd (7.5, 4.0) | 56.3 | 3.19 dd (7.5, 4.5) | 59.4 |
| 10 | 3.02 td (6.0, 4.0) | 58.2 | 3.00 td (6.0, 3.5) | 58.0 | 2.90 dd (8.5, 4.0) | 57.0 |
| 11 | 1.57 m | 27.8 | 1.54 m | 26.5 | 3.40 m | 70.0 |
| 12 | 1.39 e m | 26.7 | 1.23 e | 29.7 | 1.67 m | 34.9 |
| 13 | 1.32 e m | 29.4 | 1.29 e | 29.3 | 1.42 e | 24.8 |
| 14 | 1.25 e m | 29.5 | 1.23 e | 31.7 | 1.42 e | 29.1 |
| 15 | 1.27 e m | 32.0 | 1.26 e | 22.6 | 2.03 dd (12.5, 5.5) | 33.9 |
| 16 | 1.28 m | 22.8 | 1.46 | 27.6 | 5.76 ddd (17.0, 10.5, 6.5) | 114.7 |
| 17a | 0.88 t (7.0) | 14.3 | 0.86 t (6.5) | 14.1 | 4.90 ddd (10.0, 2.0, 1.5) | 139.0 |
| 17b | 4.96 ddd (12.0, 2.0, 1.5) | |||||
| 3-OCOCH3 | 2.08 s | 21.1 | 2.08 s | 20.7 | ||
| 3-OCOCH3 | - | 169.1 | 168.9 | |||
| 8-OCOCH3 | 2.11 s | 20.9 170.0 | 2.09 s | 20.7 169.4 | ||
| 8-OCOCH3 | - | |||||
| OMe | 3.41 s | 57.1 | ||||
| Position | 4 | panaxytriol (11) | ||
|---|---|---|---|---|
| δH (multi J in Hz) | δC | δH (multi J in Hz) | δC | |
| 1a 1b | 5.34 ddd (9.5, 1.0, 1.0) 5.54 ddd (16.5, 1.0, 1.0) | 119.8 | 5.23 ddd (10.0, 1.0, 1.0) 5.45 ddd (17.0, 1.0, 1.0) | 117.4 |
| 2 | 5.86 ddd (16.5, 10.0, 5.0) | 132.3 | 5.92 ddd (17.0, 10.0, 5.0) | 136.2 |
| 3 | 5.89 dq (5.5, 1.0) | 64.7 | 4.90 br t (5.0) | 63.6 |
| 4 | 71.4 | 75.0 | ||
| 5 | 71.6 | 71.1 | ||
| 6 | 66.6 | 66.6 | ||
| 7 | 78.6 | 78.3 | ||
| 8a 8b | 2.55 ddd (17.5, 5.5, 1.0) 2.60 ddd (17.0, 6.0, 1.0) | 25.1 | 2.55 ddd (17.5, 6.0, 1.0) 2.59 ddd (17.5, 6.0, 1.0) | 25.8 |
| 9 | 3.64 dt (5.5, 5.5) | 72.3 | 3.63 dt (5.5, 5.5) | 72.4 |
| 10 | 3.59 dt (5.5) | 73.2 | 3.57 m | 73.3 |
| 11 | 1.50 a m | 34.0 | 1.48 a m | 33.7 |
| 12a 12b | 1.35 a m 1.46 a m | 25.7 | 1.35 a m 1.45 a m | 25.1 |
| 13 | 1.27 a m | 29.7 | 1.22–1.31 | 29.8 |
| 14 | 1.27 a m | 29.4 | 29.4 | |
| 15 | 1.25 a m | 32.0 | 32.0 | |
| 16 | 1.30 a m | 22.9 | 22.9 | |
| 17 | 0.88 t (7.0) | 14.3 | 0.87 t (7.0) | 14.2 |
| 3-OCOCH3 | 2.10 s | 21.1 | ||
| 3-OCOCH3 | 169.8 | |||
| 9-OH | 2.00 br s | 2.34 d (5.0) | ||
| 10-OH | 2.40 br s | 2.74 d (5.5) | ||
| Compound | IC50 (µM) a | Compound | IC50 (µM) a | ||
|---|---|---|---|---|---|
| A2780 | SKOV3 | A2780 | SKOV3 | ||
| 1 | 11.64 ± 0.44 | >50 | 11 | 10.90 ± 0.25 | 36.38 ± 1.95 |
| 2 | 23.86 ± 0.49 | >50 | 12 | 20.88 ± 0.69 | >50 |
| 3 | >50 | >50 | 13 | 37.63 ± 1.48 | >50 |
| 4 | 25.80 ± 2.24 | >50 | 14 | >50 | >50 |
| 5 | >50 | >50 | 15 | 7.60 ± 1.33 | 27.53 ± 1.22 |
| 6 | 11.80 ± 0.81 | 43.10 ± 1.15 | 16 | 22.96 ± 0.76 | >50 |
| 7 | 20.25 ± 0.28 | >50 | 17 | >50 | >50 |
| 8 | >50 | >50 | 18 | >50 | >50 |
| 9 | 21.25 ± 0.12 | >50 | 19 | >50 | >50 |
| 10 | 22.09 ± 0.12 | >50 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, R.; Son, S.-R.; Lee, N.-K.; Kim, J.-Y.; An, G.; Choi, J.-H.; Jang, D.S. Cytotoxic Properties of C17 Polyacetylenes from the Fresh Roots of Panax ginseng on Human Epithelial Ovarian Cancer Cells. Molecules 2022, 27, 7027. https://doi.org/10.3390/molecules27207027
Kim R, Son S-R, Lee N-K, Kim J-Y, An G, Choi J-H, Jang DS. Cytotoxic Properties of C17 Polyacetylenes from the Fresh Roots of Panax ginseng on Human Epithelial Ovarian Cancer Cells. Molecules. 2022; 27(20):7027. https://doi.org/10.3390/molecules27207027
Chicago/Turabian StyleKim, Ranhee, So-Ri Son, Na-Kyung Lee, Ji-Young Kim, Gami An, Jung-Hye Choi, and Dae Sik Jang. 2022. "Cytotoxic Properties of C17 Polyacetylenes from the Fresh Roots of Panax ginseng on Human Epithelial Ovarian Cancer Cells" Molecules 27, no. 20: 7027. https://doi.org/10.3390/molecules27207027
APA StyleKim, R., Son, S. -R., Lee, N. -K., Kim, J. -Y., An, G., Choi, J. -H., & Jang, D. S. (2022). Cytotoxic Properties of C17 Polyacetylenes from the Fresh Roots of Panax ginseng on Human Epithelial Ovarian Cancer Cells. Molecules, 27(20), 7027. https://doi.org/10.3390/molecules27207027