Honey-Propolis-Engineered Collagen Peptides as Promising Wound-Healing Matrix in Mouse Model
Abstract
:1. Introduction
2. Results
2.1. Evaluation of Total Flavonoids from Extracted Propolis Wax from Bee Hive
2.2. Antioxidant Activity of HPW
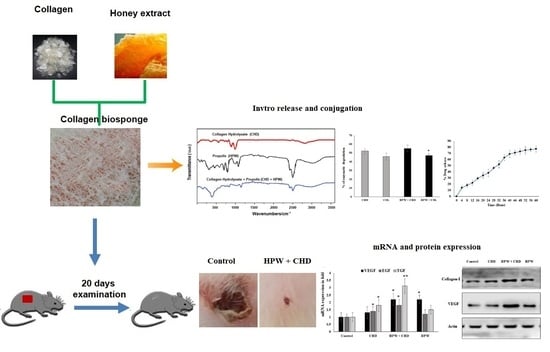
2.3. Analysis of Biosponge Fabricated with HPW
FTIR Spectroscopy
2.4. In Vivo Evaluation of Collagen Peptides (CHDs) Fabricated with HPW
3. Discussion
4. Materials and Methods
4.1. Chemicals, Cell Lines, and Reagents
4.2. Propolis Wax Extraction from a Beehive
4.3. Flavonoids Content Evaluation
In Vitro Antioxidant Assay
4.4. Hemolysis Assay
4.5. Fabrication of Collagen Sponge (CHDs) with PE
4.6. In Vitro Drug-Release Study
4.7. In Vitro Enzymatic Degradation
4.8. Assay of Cell Migration (Wound-Healing Assay)
4.9. Cell Invasion Assay
4.10. In Vivo Studies
4.11. Dressings and Surgical Procedure
4.12. Planimetry: Rate of Contraction and Period of Re-Epithelialization
4.13. Biochemical Analyses of Excised Wound Tissue
4.14. Real-Time PCR (RT-PCR)
4.15. Western Blot
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Bakil, S.N.A.; Kamal, H.; Abdullah, H.Z.; Idris, M.I. Sodium Alginate-Zinc Oxide Nanocomposite Film for Antibacterial Wound Healing Applications. Biointerface Res. Appl. Chem. 2020, 10, 6289–6296. [Google Scholar]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [Green Version]
- Tiplea, R.E.; Lemnaru, G.M.; Trusca, R.D.; Holban, A.; Kaya, M.G.A.; Dragu, L.D.; Ficai, D.; Ficai, A.; Bleotu, C. Antimicrobial Films Based on Chitosan, Collagen, and ZnO for Skin Tissue Regeneration. Biointerface Res. Appl. Chem. 2021, 11, 11985–11995. [Google Scholar]
- Frykberg, R.G.; Banks, J. Challenges in the treatment of chronic wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [Green Version]
- Muthukumar, T.; Prabu, P.; Ghosh, K.; Sastry, T.P. Fish scale collagen sponge incorporated with Macrotyloma uniflorum plant extract as a possible wound/burn dressing material. Colloids Surf. B Biointerfaces 2014, 113, 207–212. [Google Scholar] [CrossRef]
- Soleimanpour, M.; Mirhaji, S.S.; Jafari, S.; Derakhshankhah, H.; Mamashli, F.; Nedaei, H.; Karimi, M.R.; Motasadizadeh, H.; Fatahi, Y.; Ghasemi, A.; et al. Designing a new alginate-fibrinogen biomaterial composite hydrogel for wound healing. Sci. Rep. 2022, 12, 7213. [Google Scholar] [CrossRef]
- Selvaraj, S.; Fathima, N.N. Fenugreek incorporated silk fibroin nanofibers—A potential antioxidant scaffold for enhanced wound healing. ACS Appl. Mater. Interfaces 2017, 9, 5916–5926. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Gould, M.; Ali, M.A. A review of current advancements for wound healing: Biomaterial applications and medical devices. J. Biomed. Mater. Res. 2022, 110, 2542–2573. [Google Scholar] [CrossRef]
- Monti, M.; Berti, E.; Carminati, G.; Cusini, M. Occupational and cosmetic dermatitis from propolis. Contact Dermat. 1983, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Lyoussi, B.; Aazza, S.; Antunes, D.; Bankova, V.; Miguel, G. Antioxidant and α-Glucosidase Inhibitory Properties and Chemical Profiles of Moroccan Propolis. Nat. Prod. Commun. 2015, 10, 1961–1964. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, G.; Martinotti, S.; Ranzato, E. Propolis: A Multifaceted Approach for Wound Healing. In Gums, Resins and Latexes of Plant Origin: Chemistry, Biological Activities and Uses; Springer International Publishing: Cham, Switzerland, 2022; pp. 689–697. [Google Scholar]
- Marquele-Oliveira, F.; Barud, H.D.S.; Torres, E.C.; Machado, R.T.A.; Caetano, G.F.; Leite, M.N.; Frade, M.A.C.; Ribeiro, S.J.; Berretta, A.A. Development, characterization and pre-clinical trials of an innovative wound healing dressing based on propolis (EPP-AF®)-containing self-microemulsifying formulation incorporated in biocellulose membranes. Int. J. Biol. Macromol. 2019, 136, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, M.C. Propolis—Chemical composition, biological properties and therapeutic activity. Apidologie 1995, 26, 83–99. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Pi, A.; Yan, L.; Li, J.; Nan, S.; Zhang, J.; Hao, Y. Research Progress on Therapeutic Effect and Mechanism of Propolis on Wound Healing. Evid.-Based Complement. Altern. Med. 2022, 2022, 5798941. [Google Scholar] [CrossRef]
- Sehn, E.; Hernandes, L.; Franco, S.L.; Goncalves, C.C.; Baesso, M.L. Dynamics of reepithelialisation and penetration rate of a bee propolis formulation during cutaneous wounds healing. Anal. Chim. Acta. 2009, 635, 115–120. [Google Scholar] [CrossRef]
- Olczyk, P.; Mencner, Ł.; Komosinska-Vassev, K. The role of the extracellular matrix components in cutaneous wound healing. BioMed Res. Int. 2014, 2014, 747584. [Google Scholar] [CrossRef] [Green Version]
- Sutta, J.; Hanko, J.; Janda, J.; Tkac, J. Experimental and clinical experiences in the treatment of wounds in domestic animals by local application of an alcoholic solution of propolis. Bratisl. Folia Vet. 1974, 18, 143–147. [Google Scholar]
- Ennaas, N.; Hammami, R.; Beaulieu, L.; Fliss, I. Purification and characterization of four antibacterial peptides from protamex hydrolysate of Atlantic mackerel (Scomber scombrus) by-products. Biochem. Biophys. Res. Commun. 2015, 462, 195–200. [Google Scholar] [CrossRef]
- Chi, C.F.; Cao, Z.H.; Wang, B.; Hu, F.Y.; Li, Z.R.; Zhang, B. Antioxidant and functional properties of collagen hydrolysates from Spanish mackerel skin as influenced by average molecular weight. Molecules 2014, 19, 11211–11230. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, P.; Suguna, L.; Shanthi, C. Wound healing activity of a collagen-derived cryptic peptide. Amino Acids 2015, 47, 317–328. [Google Scholar] [CrossRef]
- Ramos, A.F.N.; Miranda, J.L. Propolis: A review of its anti-inflammatory and healing actions. J. Venom. Anim. Toxins Incl. Trop. Dis. 2007, 13, 698–710. [Google Scholar] [CrossRef]
- Silva, J.C.; Rodrigues, S.; Féas, X.; Estevinho, L.M. Antimicrobialactivity, phenolic profile and role in the inflammation of propolis. Food Chem. Toxicol. 2012, 50, 1790–1795. [Google Scholar] [CrossRef] [PubMed]
- de Moura, S.A.; Ferreira, M.A.; Andrade, S.P.; Reis, M.L.; Noviello Mde, L.; Cara, D.C. Brazilian green propolis inhibits inflammatory angiogenesis in a murine sponge model. Evid. Based Complement. Alternat. Med. 2011, 2011, 182703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramanathan, G.; Singaravelu, S.; Muthukumar, T.; Thyagarajan, S.; Perumal, P.T.; Sivagnanam, U.T. Design and characterization of 3D hybrid collagen matrixes as a dermal substitute in skin tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Brazil, Ministérioda Agricultura. AnexoVI-Regulamentotécnicoparaa Fixação Deidentidade Equalidadede Propolis; Ministério da Agricultura: Brasília, Brazil, 2001. [Google Scholar]
- de Almeida, E.B.; Cordeiro Cardoso, J.; Karla de Lima, A.; de Oliveira, N.L.; de Pontes-Filho, N.T.; Oliveira Lima, S.; Leal Souza, I.C.; de Albuquerque-Júnior, R.L. The incorporation of Brazilian propolis into collagen-based dressing films improves dermal burn healing. J. Ethnopharmacol. 2013, 147, 419–425. [Google Scholar] [CrossRef]
- Chang, R.; Piló-Veloso, D.; Morais, S.A.L.; Nascimento, E.A. Analysis of a Brazilian green propolis from Baccharis dracun culifolia by HPLC-APCI-MSand GC–MS. Rev. Bras. Farmacogn. 2008, 18, 549–556. [Google Scholar] [CrossRef] [Green Version]
- Silva, B.B.; Rosalen, P.L.; Cury, J.A.; Ikegaki, M.; Souza, V.C.; Esteves, A.; Alencar, S.M. Chemical composition and botanical origin of red propolis, a new type of brazilian propolis. Evid. Based Complement. Alternat. Med. 2008, 5, 313–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyyam, P.S.; Palsamy, P.; Subramanian, S.; Kandaswamy, M. Wound healing properties of Indian propolis studied on excision wound-induced rats. Pharm. Biol. 2010, 48, 1198–1206. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, M.T.; Araujo-Filho, H.G.; Barreto, A.S.; Quintans-Junior, L.J.; Quintans, J.S.; Barreto, R.S. Wound healing properties of flavonoids: A systematic review highlighting the mechanisms of action. Phytomedicine 2021, 90, 153636. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, G.; Singaravelu, S.; Raja, M.D.; Nagiah, N.; Padmapriya, P.; Ruban, K.; Kaveri, K.; Natarajan, T.S.; Sivagnanam, U.T.; Perumal, P.T. Fabrication and characterization of a collagen coated electrospun poly (3-hydroxybutyric acid)–gelatin nanofibrous scaffold as a soft bio-mimetic material for skin tissue engineering applications. RSC Adv. 2016, 6, 7914–7922. [Google Scholar] [CrossRef]
- Ramanathan, G.; Thangavelu, M.; Felciya, S.J.G.; Sivagnanam, U.T. Dual drug loaded polyhydroxy butyric acid/gelatin nanofibrous scaffold for possible post-surgery cancer treatment. Mater. Lett. 2022, 323, 132597. [Google Scholar] [CrossRef]
- Bhusnure, O.G.; Gholve, S.B.; Giram, P.S.; Gaikwad, A.V.; Udumansha, U.; Mani, G.; Tae, J.H. Novel 5-flurouracil-embedded non-woven PVA–PVP electrospun nanofibers with enhanced anti-cancer efficacy: Formulation, evaluation and in vitro anti-cancer activity. J. Drug Deliv. Sci. Technol. 2021, 64, 102654. [Google Scholar] [CrossRef]
- Martinotti, S.; Laforenza, U.; Patrone, M.; Moccia, F.; Ranzato, E. Honey-Mediated Wound Healing: H2O2 Entry through AQP3 Determines Extracellular Ca2+ Influx. Int. J. Mol. Sci. 2019, 20, 764. [Google Scholar] [CrossRef] [Green Version]
- Olczyk, P.; Komosinska-Vassev, K.; Wisowski, G.; Mencner, L.; Stojko, J.; Kozma, E.M. Propolis modulates fibronectin expression in the matrix of thermal injury. BioMed Res. Int. 2014, 2014, 748101. [Google Scholar] [CrossRef]
- da Rosa, C.; Bueno, I.L.; Quaresma AC, M.; Longato, G.B. Healing Potential of Propolis in Skin Wounds Evidenced by Clinical Studies. Pharmaceuticals 2022, 15, 1143. [Google Scholar] [CrossRef]
- Schultz, G.; Chin, G.; Moldawer, L.; Diegelmann, R. Principles of Wound Healing; University of Adelaide Press: Adelaide, Australia, 2011; Volume 23. [Google Scholar]
- Nishida, K.; Hasegawa, A.; Yamasaki, S.; Uchida, R.; Ohashi, W.; Kurashima, Y.; Kunisawa, J.; Kimura, S.; Iwanaga, T.; Watarai, H.; et al. Mast cells play role in wound healing through the ZnT2/GPR39/IL-6 axis. Sci. Rep. 2019, 9, 10842. [Google Scholar] [CrossRef] [Green Version]
- Muthukumar, T.; Senthil, R.; Sastry, T.P. Synthesis and Characterization of Biosheet Impregnated with Macrotyloma uniflorum Extract for Burn/Wound Dressings. Colloids Surf. B 2013, 102, 694–699. [Google Scholar] [CrossRef]
- Ahmed, E.A.; Alkuwayti, M.A.; Ibrahim, H.-I.M. Atropine Is a Suppressor of Epithelial–Mesenchymal Transition (EMT) That Reduces Stemness in Drug-Resistant Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 9849. [Google Scholar] [CrossRef] [PubMed]
- Negm, A.; Gouda, M.; Ibrahim, H.-I.M. Carboxymethyl Cellulose/Zn-Organic Framework Down-Regulates Proliferation and Up-Regulates Apoptosis and DNA Damage in Colon and Lung Cancer Cell Lines. Polymers 2022, 14, 2015. [Google Scholar] [CrossRef] [PubMed]
- Balthazar, J.D.; Soosaimanickam, M.P.; Emmanuel, C.; Krishnaraj, T.; Sheikh, A.; Alghafis, S.F.; Ibrahim HI, M. 8-Hydroxyquinoline a natural chelating agent from Streptomyces spp. inhibits A549 lung cancer cell lines via BCL2/STAT3 regulating pathways. World J. Microbiol. Biotechnol. 2022, 38, 182. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, T.; Prakash, D.; Anbarasu, K.; Kumar, B.S.; Sastry, T.P. Effect of collagen sponge incorporating Macrotyloma uniflorum extract on full-thickness wound healing by down-regulation of matrix metalloproteinases and inflammatory markers. RSC Adv. 2014, 4, 64267–64276. [Google Scholar] [CrossRef]







| Sample Collection | No. of Animals Used for Gross, Biochemical, and Histological Analyses | |||
|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | |
| Untreated Control | CHDs | CHD-HPW | HPW | |
| 5th day | 4 | 4 | 5 | 4 |
| 10th day | 5 | 5 | 5 | 5 |
| 15th day | 5 | 5 | 5 | 5 |
| 20th day | 5 | 5 | 6 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, H.-I.M.; Thangavelu, M.; Khalifa, A. Honey-Propolis-Engineered Collagen Peptides as Promising Wound-Healing Matrix in Mouse Model. Molecules 2022, 27, 7090. https://doi.org/10.3390/molecules27207090
Ibrahim H-IM, Thangavelu M, Khalifa A. Honey-Propolis-Engineered Collagen Peptides as Promising Wound-Healing Matrix in Mouse Model. Molecules. 2022; 27(20):7090. https://doi.org/10.3390/molecules27207090
Chicago/Turabian StyleIbrahim, Hairul-Islam Mohamed, Muthukumar Thangavelu, and Ashraf Khalifa. 2022. "Honey-Propolis-Engineered Collagen Peptides as Promising Wound-Healing Matrix in Mouse Model" Molecules 27, no. 20: 7090. https://doi.org/10.3390/molecules27207090
APA StyleIbrahim, H. -I. M., Thangavelu, M., & Khalifa, A. (2022). Honey-Propolis-Engineered Collagen Peptides as Promising Wound-Healing Matrix in Mouse Model. Molecules, 27(20), 7090. https://doi.org/10.3390/molecules27207090