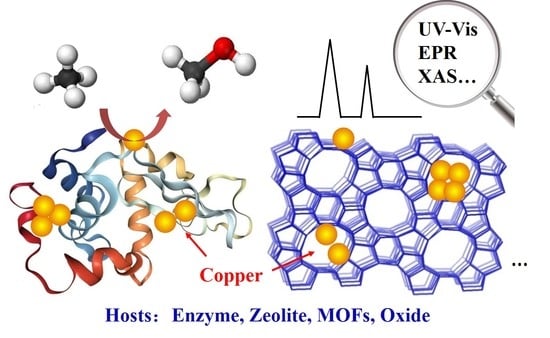
Recent Insights into Cu-Based Catalytic Sites for the Direct Conversion of Methane to Methanol
Abstract
:1. Introduction
2. Cu-Based Enzymes
2.1. The Trinuclearcopper Sites Found in Enzymes
2.2. The Dicopper Sites Found in Enzymes
2.3. The Monocopper Sites Found in Enzymes
3. Cu-Zeolites
3.1. The Dicopper Sites Supported in Zeolites
3.2. The Monocopper Sites Supported in Zeolites
3.3. The Multiple Copper Clusters Supported in Zeolites
4. Cu-MOFs
4.1. The Tricopper Sites Anchored in MOFs
4.2. The Dicopper Sites Anchored in MOFs
4.3. The Mononuclear Copper Sites Anchored in MOFs
5. Cu-Oxides
6. Catalytic Performances of Different Cu-Based Catalysts
7. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Primo, A.; Garcia, H. Zeolites as catalysts in oil refining. Chem. Soc. Rev. 2014, 43, 7548–7561. [Google Scholar] [CrossRef]
- Taarning, E.; Osmundsen, C.M.; Yang, X.; Voss, B.; Andersen, S.I.; Christensen, C.H. Zeolite-catalyzed biomass conversion to fuels and chemicals. Energy Environ. Sci. 2011, 4, 793–804. [Google Scholar] [CrossRef] [Green Version]
- Shaner, M.R.; Davis, S.J.; Lewis, N.S.; Caldeira, K. Geophysical constraints on the reliability of solar and wind power in the United States. Energ. Environ. Sci. 2018, 11, 914–925. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Cao, Q.; Wang, F.; Xiao, Y.; Li, Y.; Delaunay, J.J.; Zhu, H. Engineering graphene and TMDs based van der Waals heterostructures for photovoltaic and photoelectrochemical solar energy conversion. Chem. Soc. Rev. 2018, 47, 4981–5037. [Google Scholar] [CrossRef] [PubMed]
- Yangcheng, R.X.; Ran, J.S.; Liu, Z.H.; Cui, Y.T.; Wang, J.J. Phosphoric acid-modified commercial kieselguhr supported palladium nanoparticles as efficient catalysts for low-temperature hydrodeoxygenation of lignin derivatives in water. Green Chem. 2022, 24, 1570–1577. [Google Scholar] [CrossRef]
- Aasberg-Petersen, K.; Dybkjaer, I.; Ovesen, C.V.; Schjodt, N.C.; Sehested, J.; Thomsen, S.G. Natural gas to synthesis gas—Catalysts and catalytic processes. J. Nat. Gas Sci. Eng. 2011, 3, 423–459. [Google Scholar] [CrossRef]
- Guo, X.; Fang, G.; Li, G.; Ma, H.; Fan, H.; Yu, L.; Ma, C.; Wu, X.; Deng, D.; Wei, M.; et al. Direct, nonoxidative conversion of methane to ethylene, aromatics, and hydrogen. Science 2014, 344, 616–619. [Google Scholar] [CrossRef]
- Qi, G.; Davies, T.E.; Nasrallah, A.; Sainna, M.A.; Howe, A.G.R.; Lewis, R.J.; Quesne, M.; Catlow, C.R.A.; Willock, D.J.; He, Q.; et al. Au-ZSM-5 catalyses the selective oxidation of CH4 to CH3OH and CH3COOH using O2. Nat. Catal. 2022, 5, 45–54. [Google Scholar] [CrossRef]
- Cui, X.; Huang, R.; Deng, D. Catalytic conversion of C1 molecules under mild conditions. EnergyChem 2021, 3, 100050. [Google Scholar] [CrossRef]
- Zichittella, G.; Perez-Ramirez, J. Status and prospects of the decentralised valorisation of natural gas into energy and energy carriers. Chem. Soc. Rev. 2021, 50, 2984–3012. [Google Scholar] [CrossRef]
- Razdan, N.K.; Bhan, A. Carbidic Mo is the sole kinetically-relevant active site for catalytic methane dehydroaromatization on Mo/H-ZSM-5. J. Catal. 2020, 389, 667–676. [Google Scholar] [CrossRef]
- Morejudo, S.H.; Zanon, R.; Escolastico, S.; Yuste-Tirados, I.; Malerod-Fjeld, H.; Vestre, P.K.; Coors, W.G.; Martinez, A.; Norby, T.; Serra, J.M.; et al. Direct conversion of methane to aromatics in a catalytic co-ionic membrane reactor. Science 2016, 353, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Periana, R.A.; Taube, D.J.; Evitt, E.R.; Loffler, D.G.; Wentrcek, P.R.; Voss, G.; Masuda, T. A mercury-catalyzed, high-yield system for the oxidation of methane to methanol. Science 1993, 259, 340–343. [Google Scholar] [CrossRef]
- Luo, L.; Luo, J.; Li, H.; Ren, F.; Zhang, Y.; Liu, A.; Li, W.X.; Zeng, J. Water enables mild oxidation of methane to methanol on gold single-atom catalysts. Nat. Commun. 2021, 12, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Olsbye, U.; Svelle, S.; Bjorgen, M.; Beato, P.; Janssens, T.V.; Joensen, F.; Bordiga, S.; Lillerud, K.P. Conversion of methanol to hydrocarbons: How zeolite cavity and pore size controls product selectivity. Angew. Chem. Int. Ed. 2012, 51, 5810–5831. [Google Scholar] [CrossRef]
- Hua, J.; Dong, X.; Wang, J.; Chen, C.; Shi, Z.; Liu, Z.; Han, Y. Methanol-to-olefin conversion over small-pore DDR zeolites: Tuning the propylene selectivity via the olefin-based catalytic cycle. ACS Catal. 2020, 10, 3009–3017. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, X.; Zhu, Y.; Emwas, A.-H.; Zhang, D.; Tian, Q.; Han, Y. Investigating the influence of mesoporosity in zeolite Beta on its catalytic performance for the conversion of methanol to hydrocarbons. ACS Catal. 2015, 5, 5837–5845. [Google Scholar] [CrossRef] [Green Version]
- Usman, M.; Daud, W.M.A.W. Recent advances in the methanol synthesis via methane reforming processes. RSC Adv. 2015, 5, 21945–21972. [Google Scholar] [CrossRef]
- Kosinov, N.; Hensen, E.J.M. Reactivity, Selectivity, and Stability of Zeolite-Based Catalysts for Methane Dehydroaromatization. Adv. Mater. 2020, 32, e2002565. [Google Scholar] [CrossRef]
- Koo, C.W.; Rosenzweig, A.C. Biochemistry of aerobic biological methane oxidation. Chem. Soc. Rev. 2021, 50, 3424–3436. [Google Scholar] [CrossRef] [PubMed]
- Newton, M.A.; Knorpp, A.J.; Sushkevich, V.L.; Palagin, D.; van Bokhoven, J.A. Active sites and mechanisms in the direct conversion of methane to methanol using Cu in zeolitic hosts: A critical examination. Chem. Soc. Rev. 2020, 49, 1449–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiani, D.; Sourav, S.; Tang, Y.; Baltrusaitis, J.; Wachs, I.E. Methane activation by ZSM-5-supported transition metal centers. Chem. Soc. Rev. 2021, 50, 1251–1268. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Y.; Zhou, W.C.; Qiu, X.Y.; Li, H.D.; Jiang, Y.H.; Sun, Z.H.; Han, D.X.; Niu, L.; Tang, Z.Y. Selective photocatalytic oxidation of methane by quantum-sized bismuth vanadate. Nat. Sustain. 2021, 4, 509–515. [Google Scholar] [CrossRef]
- Feng, N.; Lin, H.; Song, H.; Yang, L.; Tang, D.; Deng, F.; Ye, J. Efficient and selective photocatalytic CH4 conversion to CH3OH with O2 by controlling overoxidation on TiO2. Nat. Commun. 2021, 12, 4652–4661. [Google Scholar] [CrossRef]
- Agarwal, N.; Freakley, S.J.; McVicker, R.U.; Althahban, S.M.; Dimitratos, N.; He, Q.; Morgan, D.J.; Jenkins, R.L.; Willock, D.J.; Taylor, S.H.; et al. Aqueous Au-Pd colloids catalyze selective CH4 oxidation to CH3OH with O2 under mild conditions. Science 2017, 358, 223–227. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.; Wang, L.; Zuidema, E.; Mondal, K.; Zhang, M.; Zhang, J.; Wang, C.; Meng, X.; Yang, H.; Mesters, C.; et al. Hydrophobic zeolite modification for in situ peroxide formation in methane oxidation to methanol. Science 2020, 367, 193–197. [Google Scholar] [CrossRef]
- Fjermestad, T.; Genest, A.; Li, W.; Mestl, G.; Roesch, N. Surface Reactivity of the Vanadium Phosphate Catalyst for the Oxidation of Methane. Top. Catal. 2017, 60, 1698–1708. [Google Scholar] [CrossRef]
- Peng, W.; Qu, X.; Shaik, S.; Wang, B. Deciphering the oxygen activation mechanism at the CuC site of particulate methane monooxygenase. Nat. Catal. 2021, 4, 266–273. [Google Scholar] [CrossRef]
- Yu, S.S.F.; Chen, K.H.C.; Tseng, M.Y.H.; Wang, Y.S.; Tseng, C.F.; Chen, Y.J.; Huang, D.S.; Chan, S.I. Production of high-quality particulate methane monooxygenase in high yields from Methylococcus capsulatus (Bath) with a hollow-fiber membrane bioreactor. J. Bacteriol. 2003, 185, 5915–5924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieberman, R.L.; Shrestha, D.B.; Doan, P.E.; Hoffman, B.M.; Stemmler, T.L.; Rosenzweig, A.C. Purified particulate methane monooxygenase from Methylococcus capsulatus (Bath) is a dimer with both mononuclear copper and a copper-containing cluster. Proc. Natl. Acad. Sci. USA 2003, 100, 3820–3825. [Google Scholar] [CrossRef]
- Chan, S.I.; Chen, K.H.C.; Yu, S.S.F.; Chen, C.L.; Kuo, S.S.J. Toward delineating the structure and function of the particulate methane monooxygenase from methanotrophic bacteria. Biochemistry 2004, 43, 4421–4430. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.I.; Wang, V.C.; Lai, J.C.; Yu, S.S.; Chen, P.P.; Chen, K.H.; Chen, C.L.; Chan, M.K. Redox potentiometry studies of particulate methane monooxygenase: Support for a trinuclear copper cluster active site. Angew. Chem. Int. Ed. 2007, 46, 1992–1994. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Wu, C.Q.; Sung, P.H.; Chan, S.I.; Chen, P.P.Y. Turnover of a Methane Oxidation Tricopper Cluster Catalyst: Implications for the Mechanism of the Particulate Methane Monooxygenase (pMMO). Chemcatchem 2020, 12, 3088–3096. [Google Scholar] [CrossRef]
- Lieberman, R.L.; Rosenzweig, A.C. Crystal structure of a membrane-bound metalloenzyme that catalyses the biological oxidation of methane. Nature 2005, 434, 177–182. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Smith, S.M.; Rawat, S.; Yatsunyk, L.A.; Stemmler, T.L.; Rosenzweig, A.C. Oxidation of methane by a biological dicopper centre. Nature 2010, 465, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.M.; Rawat, S.; Telser, J.; Hoffman, B.M.; Stemmler, T.L.; Rosenzweig, A.C. Crystal Structure and Characterization of Particulate Methane Monooxygenase from Methylocystis species Strain M. Biochemistry 2011, 50, 10231–10240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, M.O.; MacMillan, F.; Wang, J.; Nisthal, A.; Lawton, T.J.; Olafson, B.D.; Mayo, S.L.; Rosenzweig, A.C.; Hoffman, B.M. Particulate methane monooxygenase contains only mononuclear copper centers. Science 2019, 364, 566–570. [Google Scholar] [CrossRef] [Green Version]
- Ro, S.Y.; Schachner, L.F.; Koo, C.W.; Purohit, R.; Remis, J.P.; Kenney, G.E.; Liauw, B.W.; Thomas, P.M.; Patrie, S.M.; Kelleher, N.L.; et al. Native top-down mass spectrometry provides insights into the copper centers of membrane-bound methane monooxygenase. Nat. Commun. 2019, 10, 2675–2686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jodts, R.J.; Ross, M.O.; Koo, C.W.; Doan, P.E.; Rosenzweig, A.C.; Hoffman, B.M. Coordination of the Copper Centers in Particulate Methane Monooxygenase: Comparison between Methanotrophs and Characterization of the CuC Site by EPR and ENDOR Spectroscopies. J. Am. Chem. Soc. 2021, 143, 15358–15368. [Google Scholar] [CrossRef]
- Davydov, R.; Herzog, A.E.; Jodts, R.J.; Karlin, K.D.; Hoffman, B.M. End-On Copper(I) Superoxo and Cu(II) Peroxo and Hydroperoxo Complexes Generated by Cryoreduction/Annealing and Characterized by EPR/ENDOR Spectroscopy. J. Am. Chem. Soc. 2022, 144, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Lawton, T.J.; Rosenzweig, A.C. Methane-Oxidizing Enzymes: An Upstream Problem in Biological Gas-to-Liquids Conversion. J. Am. Chem. Soc. 2016, 138, 9327–9340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, W.H.; Lin, H.H.; Tsai, I.K.; Huang, S.H.; Chung, S.C.; Tu, I.P.; Yu, S.S.; Chan, S.I. Copper Centers in the Cryo-EM Structure of Particulate Methane Monooxygenase Reveal the Catalytic Machinery of Methane Oxidation. J. Am. Chem. Soc. 2021, 143, 9922–9932. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hua, Y.; Wang, J.; Dong, X.; Tian, Q.; Han, Y. Recent progress in the direct synthesis of hierarchical zeolites: Synthetic strategies and characterization methods. Mater. Chem. Front. 2017, 1, 2195–2212. [Google Scholar] [CrossRef]
- Del Campo, P.; Martinez, C.; Corma, A. Activation and conversion of alkanes in the confined space of zeolite-type materials. Chem. Soc. Rev. 2021, 50, 8511–8595. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Dai, W.; Wu, G.; Guan, N.; Li, L. Confinement in a Zeolite and Zeolite Catalysis. Acc. Chem. Res. 2021, 54, 2894–2904. [Google Scholar] [CrossRef] [PubMed]
- Smeets, P.J.; Hadt, R.G.; Woertink, J.S.; Vanelderen, P.; Schoonheydt, R.A.; Sels, B.F.; Solomon, E.I. Oxygen precursor to the reactive intermediate in methanol synthesis by Cu-ZSM-5. J. Am. Chem. Soc. 2010, 132, 14736–14738. [Google Scholar] [CrossRef] [Green Version]
- Pappas, D.K.; Martini, A.; Dyballa, M.; Kvande, K.; Teketel, S.; Lomachenko, K.A.; Baran, R.; Glatzel, P.; Arstad, B.; Berlier, G.; et al. The nuclearity of the active site for methane to methanol conversion in Cu-mordenite: A quantitative assessment. J. Am. Chem. Soc. 2018, 140, 15270–15278. [Google Scholar] [CrossRef]
- Vanelderen, P.; Snyder, B.E.; Tsai, M.L.; Hadt, R.G.; Vancauwenbergh, J.; Coussens, O.; Schoonheydt, R.A.; Sels, B.F.; Solomon, E.I. Spectroscopic definition of the copper active sites in mordenite: Selective methane oxidation. J. Am. Chem. Soc. 2015, 137, 6383–6392. [Google Scholar] [CrossRef] [Green Version]
- Sushkevich, V.L.; Palagin, D.; Ranocchiari, M.; van Bokhoven, J.A. Selective anaerobic oxidation of methane enables direct synthesis of methanol. Science 2017, 356, 523–527. [Google Scholar] [CrossRef]
- Sushkevich, V.L.; Palagin, D.; van Bokhoven, J.A. The effect of the active-site structure on the activity of copper mordenite in the aerobic and anaerobic conversion of methane into methanol. Angew. Chem. Int. Ed. 2018, 57, 8906–8910. [Google Scholar] [CrossRef]
- Sushkevich, V.L.; Verel, R.; van Bokhoven, J.A. Pathways of methane transformation over copper-exchanged mordenite as revealed by in situ NMR and IR spectroscopy. Angew. Chem. Int. Ed. 2020, 59, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Knorpp, A.J.; Pinar, A.B.; Baerlocher, C.; McCusker, L.B.; Casati, N.; Newton, M.A.; Checchia, S.; Meyet, J.; Palagin, D.; Bokhoven, J.A. Paired copper monomers in zeolite omega: The active site for methane-to-methanol conversion. Angew. Chem. Int. Ed. 2021, 60, 5854–5858. [Google Scholar] [CrossRef]
- Ipek, B.; Wulfers, M.J.; Kim, H.; Goltl, F.; Hermans, I.; Smith, J.P.; Booksh, K.S.; Brown, C.M.; Lobo, R.F. Formation of [Cu2O2]2+ and [Cu2O]2+ toward C-H bond activation in Cu-SSZ-13 and Cu-SSZ-39. ACS Catal. 2017, 7, 4291–4303. [Google Scholar] [CrossRef]
- Sun, L.L.; Wang, Y.; Wang, C.M.; Xie, Z.K.; Guan, N.J.; Li, L.D. Water-involved methane-selective catalytic oxidation by dioxygen over copper zeolites. Chem 2021, 7, 1557–1568. [Google Scholar] [CrossRef]
- Grundner, S.; Markovits, M.A.; Li, G.; Tromp, M.; Pidko, E.A.; Hensen, E.J.; Jentys, A.; Sanchez-Sanchez, M.; Lercher, J.A. Single-site trinuclear copper oxygen clusters in mordenite for selective conversion of methane to methanol. Nat. Commun. 2015, 6, 7546–7554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Vassilev, P.; Sanchez-Sanchez, M.; Lercher, J.A.; Hensen, E.J.M.; Pidko, E.A. Stability and reactivity of copper oxo-clusters in ZSM-5 zeolite for selective methane oxidation to methanol. J. Catal. 2016, 338, 305–312. [Google Scholar] [CrossRef]
- Mahyuddin, M.H.; Tanaka, T.; Shiota, Y.; Staykov, A.; Yoshizawa, K. Methane Partial Oxidation over [Cu2(μ-O)]2+ and [Cu3(μ-O)3]2+ Active Species in Large-Pore Zeolites. ACS Catal. 2018, 8, 1500–1509. [Google Scholar] [CrossRef]
- Palagin, D.; Knorpp, A.J.; Pinar, A.B.; Ranocchiari, M.; van Bokhoven, J.A. Assessing the relative stability of copper oxide clusters as active sites of a CuMOR zeolite for methane to methanol conversion: Size matters? Nanoscale 2017, 9, 1144–1153. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Q.; Jiang, H.L. Metal-organic frameworks meet metal nanoparticles: Synergistic effect for enhanced catalysis. Chem. Soc. Rev. 2017, 46, 4774–4808. [Google Scholar] [CrossRef]
- Ikuno, T.; Zheng, J.; Vjunov, A.; Sanchez-Sanchez, M.; Ortuno, M.A.; Pahls, D.R.; Fulton, J.L.; Camaioni, D.M.; Li, Z.; Ray, D.; et al. Methane oxidation to methanol catalyzed by Cu-oxo clusters stabilized in NU-1000 metal-organic framework. J. Am. Chem. Soc. 2017, 139, 10294–10301. [Google Scholar] [CrossRef]
- Zheng, J.; Ye, J.; Ortuno, M.A.; Fulton, J.L.; Gutierrez, O.Y.; Camaioni, D.M.; Motkuri, R.K.; Li, Z.; Webber, T.E.; Mehdi, B.L.; et al. Selective Methane Oxidation to Methanol on Cu-Oxo Dimers Stabilized by Zirconia Nodes of an NU-1000 Metal-Organic Framework. J. Am. Chem. Soc. 2019, 141, 9292–9304. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Rungtaweevoranit, B.; Pei, X.; Park, M.; Fakra, S.C.; Liu, Y.S.; Matheu, R.; Alshmimri, S.A.; Alshehri, S.; Trickett, C.A.; et al. Bioinspired Metal-Organic Framework Catalysts for Selective Methane Oxidation to Methanol. J. Am. Chem. Soc. 2018, 140, 18208–18216. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kwon, C.; Keum, C.; Kim, H.E.; Lee, H.; Han, B.; Lee, S.Y. Methane partial oxidation by monomeric Cu active center confined on ZIF-7. Chem. Eng. J. 2022, 450, 138472. [Google Scholar] [CrossRef]
- Bozbag, S.E.; Sot, P.; Nachtegaal, M.; Ranocchiari, M.; van Bokhoven, J.A.; Mesters, C. Direct stepwise oxidation of methane to methanol over Cu–SiO2. ACS Catal. 2018, 8, 5721–5731. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Huang, E.; Orozco, I.; Liao, W.; Palomino, R.M.; Rui, N.; Duchon, T.; Nemsak, S.; Grinter, D.C.; Mahapatra, M.; et al. Water-promoted interfacial pathways in methane oxidation to methanol on a CeO2-Cu2O catalyst. Science 2020, 368, 513–517. [Google Scholar] [CrossRef]
- Zuo, Z.; Ramirez, P.J.; Senanayake, S.D.; Liu, P.; Rodriguez, J.A. Low-Temperature Conversion of Methane to Methanol on CeOx/Cu2O Catalysts: Water Controlled Activation of the C-H Bond. J. Am. Chem. Soc. 2016, 138, 13810–13813. [Google Scholar] [CrossRef]
- Lustemberg, P.G.; Palomino, R.M.; Gutierrez, R.A.; Grinter, D.C.; Vorokhta, M.; Liu, Z.; Ramirez, P.J.; Matolin, V.; Ganduglia-Pirovano, M.V.; Senanayake, S.D.; et al. Direct conversion of methane to methanol on Ni-ceria surfaces: Metal-support interactions and water-enabled catalytic conversion by site blocking. J. Am. Chem. Soc. 2018, 140, 7681–7687. [Google Scholar] [CrossRef]
- Huang, E.; Orozco, I.; Ramirez, P.J.; Liu, Z.; Zhang, F.; Mahapatra, M.; Nemsak, S.; Senanayake, S.D.; Rodriguez, J.A.; Liu, P. Selective Methane Oxidation to Methanol on ZnO/Cu2O/Cu(111) Catalysts: Multiple Site-Dependent Behaviors. J. Am. Chem. Soc. 2021, 143, 19018–19032. [Google Scholar] [CrossRef]











| Catalyst | Active Sites | Oxidant | Activation Temperature (°C) | Reaction Temperature (°C) | Reaction Rate a (molCH3OH/molCu) | Ref. |
|---|---|---|---|---|---|---|
| pMMO | Dicopper | Air | 37 | 37 | 5.3 nmol/(min mg) | [36] |
| pMMO | Monocopper | Air | 45 | 45 | 9 µM/h | [37] |
| pMMO | Monocopper | Air | 35 | 35 | 17 µM/12 h | [37] |
| Cu-ZSM-5 | [Cu2-(µ-O)2]2+[Cu2-(µ-O)2]2+ | O2 | 450 | 100 | 0.029/cycle | [21] |
| Cu-MOR | O2 | 450 | 100 | 0.018/cycle | [21] | |
| Cu/SiO2 | / | O2 | 450 | 100 | 0.003/cycle | [21] |
| Cu-ZSM-5 | [Cu2O]2+ | O2 | 450 | 100 | / | [46] |
| Cu-MOR | [Cu2O]2+ | H2O | 400 | 200 | 0.204/cycle | [49] |
| Cu-MOR | [Cu2O]2+ | O2 | 500 | 200 | 0.47/cycle | [47] |
| Cu-MOR(6.5) | [Cu2O]2+ | O2 | 400 | 200 | 0.142cycle | [50] |
| Cu-MOR(10) | [Cu2O]2+ | O2 | 400 | 200 | 0.216/cycle | [50] |
| Cu-MOR(46) | [CuOH]+ | O2 | 400 | 200 | 0.316/cycle | [50] |
| Cu-MOR(6.5) | [Cu2O]2+ | H2O | 400 | 200 | 0.204/cycle | [50] |
| Cu-MOR(10) | [Cu2O]2+ | H2O | 400 | 200 | 0.187/cycle | [50] |
| Cu-MOR(46) | [CuOH]+ | H2O | 400 | 200 | 0/cycle | [50] |
| Cu-omega | [CuOH]+ | O2 | 450 | 200 | 0.22/cycle | [52] |
| Cu-CHA | CuOOH | O2/H2O | 300 | 300 | 0.543/h | [54] |
| Cu-NU-1000 | Tricopper | O2 | 200 | 150 | 17.7 µmol/g | [60] |
| Cu/MOF-808 | Dicopper | N2O | 150 | 150 | 71.8 µmol/g | [62] |
| Cu-ZIF-7 | Monocopper | H2O2 | 50 | 50 | ~100 µmol/g | [63] |
| Cu/SiO2 | [Cu2O]2+ | O2 | 500 | 200 | 0.016/cycle | [64] |
| Cu/SiO2 | [Cu2O]2+ | O2 | 800 | 200 | 0.037/cycle | [64] |
| CeO2/Cu2O/Cu | / | O2/H2O | 177 | 177 | 70% Sel.b | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, M.; Liu, L.; Liu, Z. Recent Insights into Cu-Based Catalytic Sites for the Direct Conversion of Methane to Methanol. Molecules 2022, 27, 7146. https://doi.org/10.3390/molecules27217146
Mao M, Liu L, Liu Z. Recent Insights into Cu-Based Catalytic Sites for the Direct Conversion of Methane to Methanol. Molecules. 2022; 27(21):7146. https://doi.org/10.3390/molecules27217146
Chicago/Turabian StyleMao, Min, Lingmei Liu, and Zhaohui Liu. 2022. "Recent Insights into Cu-Based Catalytic Sites for the Direct Conversion of Methane to Methanol" Molecules 27, no. 21: 7146. https://doi.org/10.3390/molecules27217146
APA StyleMao, M., Liu, L., & Liu, Z. (2022). Recent Insights into Cu-Based Catalytic Sites for the Direct Conversion of Methane to Methanol. Molecules, 27(21), 7146. https://doi.org/10.3390/molecules27217146