Correlation of Phosphorus Adsorption with Chemical Properties of Aluminum-Based Drinking Water Treatment Residuals Collected from Various Parts of the United States
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical Properties and Toxicity Analysis of Al-WTRs
2.3. Phosphorus Adsorption Experiments
3. Results and Discussion
3.1. Metal Concentrations and Toxicity Analysis of Al-WTRs
3.2. Chemical Properties of Al-WTRs
3.3. Phosphorus Adsorption on Al-WTRs
3.4. Correlation of P Adsorption with Chemical Properties of Al-WTRs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nielsen, S.S.; Kjeldsen, P.; Jakobsen, R. Full scale amendment of a contaminated wood impregnation site with iron water treatment residues. Front. Environ. Sci. Eng. 2016, 10, 3. [Google Scholar] [CrossRef] [Green Version]
- Ippolito, J.A.; Barbarick, K.A.; Elliott, H.A. Drinking water treatment residuals: A review of recent uses. J. Environ. Qual. 2011, 40, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Lu, J.; Dopson, M.; Leiviskä, T. Vanadium removal from mining ditch water using commercial iron products and ferric groundwater treatment residual-based materials. Chemosphere 2022, 286, 131817. [Google Scholar] [CrossRef]
- Zhao, Y.; Wendling, L.A.; Wang, C.; Pei, Y. Use of Fe/Al drinking water treatment residuals as amendments for enhancing the retention capacity of glyphosate in agricultural soils. J. Environ. Sci. 2015, 34, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Na Nagara, V.; Sarkar, D.; Barrett, K.; Datta, R. Greening the gray infrastructure: Green adsorbent media for catch basin inserts to remove stormwater pollutants. Environ. Technol. Innov. 2021, 21, 101334. [Google Scholar] [CrossRef]
- Na Nagara, V.; Sarkar, D.; Elzinga, E.J.; Datta, R. Removal of heavy metals from stormwater runoff using granulated drinking water treatment residuals. Environ. Technol. Innov. 2022, 28, 102636. [Google Scholar] [CrossRef]
- Wang, M.; Bai, S.; Wang, X. Enhanced removal of heavy metals and phosphate in stormwater filtration systems amended with drinking water treatment residual-based granules. J. Environ. Manag. 2021, 280, 111645. [Google Scholar] [CrossRef]
- Zhou, L.; Wallace, S.M.; Denslow, N.D.; Gaillard, J.F.; Meyer, P.; Bonzongo, J.C.J. A Screening Approach for the Selection of Drinking Water Treatment Residuals for Their Introduction to Marine Systems. Environ. Toxicol. Chem. 2021, 40, 1194–1203. [Google Scholar] [CrossRef]
- El-Kammah, M.; Elkhatib, E.; Gouveia, S.; Cameselle, C.; Aboukila, E. Cost-effective ecofriendly nanoparticles for rapid and efficient indigo carmine dye removal from wastewater: Adsorption equilibrium, kinetics and mechanism. Environ. Technol. Innov. 2022, 28, 102595. [Google Scholar] [CrossRef]
- Liu, H.; Huang, P.; Liang, Z.; Zhao, Z.; Cui, F. Selective adsorption of anions on hydrotalcite-like compounds derived from drinking water treatment residuals. Chemosphere 2022, 300, 134508. [Google Scholar] [CrossRef]
- Cui, X.; Liu, X.; Lin, C.; He, M.; Ouyang, W. Activation of peroxymonosulfate using drinking water treatment residuals modified by hydrothermal treatment for imidacloprid degradation. Chemosphere 2020, 254, 126820. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Cui, X.; Zhang, H.; Liu, X.; Lin, C.; He, M.; Ouyang, W. Efficient catalyst prepared from water treatment residuals and industrial glucose using hydrothermal treatment: Preparation, characterization and its catalytic performance for activating peroxymonosulfate to degrade imidacloprid. Chemosphere 2022, 290, 133326. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Huang, X.; Lin, C.; He, M.; Ouyang, W. Activation of peroxymonosulfate by WTRs-based iron-carbon composites for atrazine removal: Performance evaluation, mechanism insight and byproduct analysis. Chem. Eng. J. 2020, 421, 127811. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Ma, J.; Lin, C.; Qi, C.; Li, X.; Zhou, Z.; Fan, G. Activation of peroxymonosulfate using drinking water treatment residuals for the degradation of atrazine. J. Hazard. Mater. 2018, 344, 1220–1228. [Google Scholar] [CrossRef]
- Qi, C.; Yu, G.; Huang, J.; Wang, B.; Wang, Y.; Deng, S. Activation of persulfate by modified drinking water treatment residuals for sulfamethoxazole degradation. Chem. Eng. J. 2018, 353, 490–498. [Google Scholar] [CrossRef]
- Turner, T.; Wheeler, R.; Stone, A.; Oliver, I. Potential Alternative Reuse Pathways for Water Treatment Residuals: Remaining Barriers and Questions—A Review. Water Air Soil Pollut. 2019, 230, 9. [Google Scholar] [CrossRef] [Green Version]
- Pachana, P.K.; Rattanasak, U.; Nuithitikul, K.; Jitsangiam, P.; Chindaprasirt, P. Sustainable utilization of water treatment residue as a porous geopolymer for iron and manganese removals from groundwater. J. Environ. Manag. 2022, 302, 114036. [Google Scholar] [CrossRef]
- Ackah, L.A.; Guru, R.; Peiravi, M.; Mohanty, M.; Ma, X.; Kumar, S.; Liu, J. Characterization of Southern Illinois water treatment residues for sustainable applications. Sustainability 2018, 10, 1374. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Liu, R.; Awe, O.W.; Yang, Y.; Shen, C. Acceptability of land application of alum-based water treatment residuals–An explicit and comprehensive review. Chem. Eng. J. 2018, 353, 717–726. [Google Scholar] [CrossRef]
- Ippolito, J.A. Aluminum-Based Water Treatment Residual Use in a Constructed Wetland for Capturing Urban Runoff Phosphorus: Column Study. Water Air Soil Pollut. 2015, 226, 334. [Google Scholar] [CrossRef]
- Banet, T.; Massey, M.S.; Zohar, I.; Litaor, M.I.; Ippolito, J.A. Phosphorus removal from swine wastewater using aluminum-based water treatment residuals. Resour. Conserv. Recycl. X 2020, 6, 100039. [Google Scholar] [CrossRef]
- Shen, C.; Zhao, Y.; Li, W.; Yang, Y.; Liu, R.; Morgen, D. Global profile of heavy metals and semimetals adsorption using drinking water treatment residual. Chem. Eng. J. 2019, 372, 1019–1027. [Google Scholar] [CrossRef]
- Xu, D.; Lee, L.Y.; Lim, F.Y.; Lyu, Z.; Zhu, H.; Ong, S.L.; Hu, J. Water treatment residual: A critical review of its applications on pollutant removal from stormwater runoff and future perspectives. J. Environ. Manag. 2020, 259, 109649. [Google Scholar] [CrossRef]
- Callery, O.; Healy, M.G. A novel method to rapidly assess the suitability of water treatment residual and crushed concrete for the mitigation of point and nonpoint source nutrient pollution. Resour. Conserv. Recycl. X 2019, 2, 100010. [Google Scholar] [CrossRef]
- USEPA. Indicators: Phosphorus|National Aquatic Resource Surveys 2021. Available online: https://www.epa.gov/national-aquatic-resource-surveys/indicators-phosphorus (accessed on 31 August 2022).
- Griffiths, L.N.; Mitsch, W.J. Removal of nutrients from urban stormwater runoff by storm-pulsed and seasonally pulsed created wetlands in the subtropics. Ecol. Eng. 2017, 108, 414–424. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, S.; Wang, J.; Ding, X. Phosphorus retention using iron (II/III) modified biochar in saline-alkaline soils: Adsorption, column and field tests. Environ. Pollut. 2020, 261, 114223. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Method 1312 Synthetic Precipitation Leaching Procedure; EPA: Washington, DC, USA, 1994.
- USEPA. Method 3050B Acid Digestion of Sediments, Sludges, and Soils 1.0 Scope and Application; EPA: Washington, DC, USA, 1996.
- Darke, A.K. Changes in Al and Fe crystallinity and P Sorption capacity in a flood-plain forest soil subjected to artificially manipulated flooding regimes in field mesocosms. Wetl. Ecol. Manag. 1996, 4, 235–244. [Google Scholar] [CrossRef]
- Dayton, E.A.; Basta, N.T. A Method for Determining the Phosphorus Sorption Capacity and Amorphous Aluminum of Aluminum-Based Drinking Water Treatment Residuals. J. Environ. Qual. 2005, 34, 1112–1118. [Google Scholar] [CrossRef] [Green Version]
- Castaldi, P.; Mele, E.; Silvetti, M.; Garau, G.; Deiana, S. Water treatment residues as accumulators of oxoanions in soil. Sorption of arsenate and phosphate anions from an aqueous solution. J. Hazard. Mater. 2014, 264, 144–152. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Jin, Z.; Guo, J.; Yang, H.; Zeng, Y.; Liu, Y. Simultaneous removal of phosphate and ammonium nitrogen from agricultural runoff by amending soil in lakeside zone of Karst area, Southern China. Agric. Ecosyst. Environ. 2020, 289, 106745. [Google Scholar] [CrossRef]
- Pourhakkak, P.; Taghizadeh, A.; Taghizadeh, M.; Ghaedi, M.; Haghdoust, S. Fundamentals of adsorption technology. Interface Sci. Technol. 2021, 33, 1–70. [Google Scholar] [CrossRef]
- Li, X.; Cui, J.; Pei, Y. Granulation of drinking water treatment residuals as applicable media for phosphorus removal. J. Environ. Manag. 2018, 213, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Su, L.; Pei, Y. Synthesis and toxic metals (Cd, Pb, and Zn) immobilization properties of drinking water treatment residuals and metakaolin-based geopolymers. Mater. Chem. Phys. 2020, 242, 122535. [Google Scholar] [CrossRef]
- Sidhu, V.; Barrett, K.; Park, D.Y.; Deng, Y.; Datta, R.; Sarkar, D. Wood mulch coated with iron-based water treatment residuals for the abatement of metals and phosphorus in simulated stormwater runoff. Environ. Technol. Innov. 2020, 21, 101214. [Google Scholar] [CrossRef]
- Soleimanifar, H.; Deng, Y.; Wu, L.; Sarkar, D. Water treatment residual (WTR)-coated wood mulch for alleviation of toxic metals and phosphorus from polluted urban stormwater runoff. Chemosphere 2016, 154, 289–292. [Google Scholar] [CrossRef]
- Bai, L.; Wang, C.; He, L.; Pei, Y. Influence of the inherent properties of drinking water treatment residuals on their phosphorus adsorption capacities. J. Environ. Sci. 2014, 26, 2397–2405. [Google Scholar] [CrossRef]
- Agyin-Birikorang, S.; O’Connor, G.A. Lability of drinking water treatment residuals (WTR) immobilized phosphorus: Aging and pH effects. J. Environ. Qual. 2007, 36, 1076–1085. [Google Scholar] [CrossRef] [Green Version]
- Norris, M.; Titshall, L. The distribution of inherent phosphorous in fifteen water treatment residues from South Africa. Water SA 2012, 38, 715–720. [Google Scholar] [CrossRef] [Green Version]
- Olatunji, O.O.; Oyeyiola, Y.; Oyediran, G.O. Assessment of Dithionite and Oxalate Extractable Iron and Aluminium Oxides on a Landscape on Basement Complex Soil in South-Western Nigeria. Open J. Soil Sci. 2015, 5, 266–275. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Bol, R.; Willbold, S.; Vereecken, H.; Klumpp, E. Speciation and distribution of P associated with Fe and Al oxides Speciation and distribution of P associated with Fe and Al oxides in aggregate-sized fraction of an arable soil. Biogeosciences 2015, 12, 9879–9903. [Google Scholar] [CrossRef]
- Babatunde, A.O.; Zhao, Y.Q.; Burke, A.M.; Morris, M.A.; Hanrahan, J.P. Characterization of aluminium-based water treatment residual for potential phosphorus removal in engineered wetlands. Environ. Pollut. 2009, 157, 2830–2836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Q.; Meng, P.; Pei, H.; Hu, W.; Chen, Y. Phosphorus adsorption characteristics of alum sludge: Adsorption capacity and the forms of phosphorus retained in alum sludge. Mater. Lett. 2018, 229, 31–35. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.Q.; Babatunde, A.O.; Wang, L.; Ren, Y.X.; Han, Y. Characteristics and mechanisms of phosphate adsorption on dewatered alum sludge. Sep. Purif. Technol. 2006, 51, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Gypser, S.; Hirsch, F.; Schleicher, A.M.; Freese, D. Impact of crystalline and amorphous iron- and aluminum hydroxides on mechanisms of phosphate adsorption and desorption. J. Environ. Sci. 2018, 70, 175–189. [Google Scholar] [CrossRef]
- Bal Krishna, K.C.; Aryal, A.; Jansen, T. Comparative study of ground water treatment plants sludges to remove phosphorous from wastewater. J. Environ. Manag. 2016, 180, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Phillips, B.L.; Boily, J.F.; Hu, Y.; Hu, Z.; Yang, P.; Feng, X.; Xu, W.; Zhu, M. Phosphate Sorption Speciation and Precipitation Mechanisms on Amorphous Aluminum Hydroxide. Soil Syst. 2019, 3, 20. [Google Scholar] [CrossRef] [Green Version]
- Maqbool, N.; Khan, Z.; Asghar, A. Reuse of alum sludge for phosphorus removal from municipal wastewater. Desalination Water Treat. 2016, 57, 13246–13254. [Google Scholar] [CrossRef]
- Gibbons, M.K.; Mortula, M.M.; Gagnon, G.A. Phosphorus adsorption on water treatment residual solids. J. Water Supply Res. Technol. AQUA 2009, 58, 1–10. [Google Scholar] [CrossRef]
- Carleton, G.; Al daach, H.; Cutright, T.J. Laboratory evaluation of alum, ferric and ferrous-water treatment residuals for removing phosphorous from surface water. Heliyon 2020, 6, e04681. [Google Scholar] [CrossRef]
- Gao, S.; Wang, C.; Pei, Y. Comparison of different phosphate species adsorption by ferric and alum water treatment residuals. J. Environ. Sci. 2013, 25, 986–992. [Google Scholar] [CrossRef]
- Makris, K.C.; El-Shall, H.; Harris, W.G.; O’Connor, G.A.; Obreza, T.A. Intraparticle phosphorus diffusion in a drinking water treatment residual at room temperature. J. Colloid Interface Sci. 2004, 277, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Muisa, N.; Nhapi, I.; Ruziwa, W.; Manyuchi, M.M. Utilization of alum sludge as adsorbent for phosphorus removal in municipal wastewater: A review. J. Water Process Eng. 2020, 35, 101187. [Google Scholar] [CrossRef]
- Hair, J.; William, C.; Black, B.; Babin, J.; Rolph, E.A. Multivariate Data Analysis, 7th ed.; Pearson: London, UK, 2009. [Google Scholar]



| Sample | NJ | MO | SD | NY | FL | OK | NE | KY | ME | CA | NC | MA | CT | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| USEPA Limit | |||||||||||||||
| TCLP Values (mg/L) | As | 5 | 1.9 | 0.12 | 0.8 | 0.88 | 1.12 | 2.28 | 2.11 | 1.48 | 1.88 | 3.23 | 1.58 | 1.65 | 1.95 |
| Ba | 100 | 1.44 | 2.25 | 0.46 | 2.02 | 0.27 | 3.25 | 1.43 | 2.39 | 0.1 | 1.88 | BDL 1 | BDL | BDL | |
| Cd | 1 | 0.03 | 0.01 | BDL | 0.01 | BDL | BDL | BDL | BDL | BDL | BDL | 0.03 | 0.04 | 0.04 | |
| Cr | 5 | 0.02 | 0.01 | 0.01 | BDL | 0.07 | 0.04 | 0.05 | 0.04 | 0.01 | 0.04 | 0.02 | 0.18 | 0.25 | |
| Pb | 5 | 0.24 | 0.04 | 0.01 | 0.07 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.29 | 0.3 | 0.35 | |
| Hg | 0.2 | BDL | BDL | 0.01 | BDL | BDL | BDL | 0.01 | BDL | BDL | BDL | BDL | BDL | BDL | |
| Se | 2 | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL | |
| Ag | 5 | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL | |
| Cu | NS 2 | 0.05 | 0.06 | BDL | BDL | 1.2 | 1.2 | 1.2 | 1.3 | 1.3 | 1.3 | 0 | 0.02 | 0.12 | |
| Ni | NS | BDL | 0.06 | BDL | 0.03 | BDL | BDL | BDL | BDL | BDL | BDL | BDL | 0.06 | 0.02 | |
| Zn | NS | 0.24 | 0.19 | 0.01 | 0.04 | BDL | 0.02 | BDL | 0.13 | 0.46 | 0.5 | 0.04 | 0.27 | 0.39 | |
| EC (mS/cm) | 0.63 | 1.72 | 0.98 | 0.88 | 1.1 | 1.08 | 0.04 | 0.96 | 0.02 | 0.004 | 0.13 | 0.7 | 1.23 | ||
| pH | 6.08 | 7.3 | 9.79 | 7.47 | 8.47 | 9.4 | 7.75 | 7.39 | 6.36 | 7.05 | 6.03 | 7.27 | 6.71 | ||
| OM (%) | 14.31 | 0.45 | 2.32 | 1.17 | 0.11 | 0.29 | 0.16 | 3.66 | 7.03 | 5.84 | 3.37 | 1.8 | 2.11 | ||
| C (%) | 8.24 | 3.91 | 10.64 | 6.26 | 12.29 | 9.89 | 11.33 | 12.04 | 17.15 | 10.03 | 3.81 | 7.61 | 11.7 | ||
| N (%) | 0.78 | 0.47 | 0.21 | 0.64 | 0.12 | 0.17 | 0.12 | 0.91 | 0.96 | 1.33 | 0.74 | 0.8 | 1.04 | ||
| S (%) | 8.13 | 1.68 | 0.33 | 4.14 | 0.35 | 0.32 | 0.33 | 5.45 | 13.43 | 9.6 | 0.49 | 0.74 | 1.65 | ||
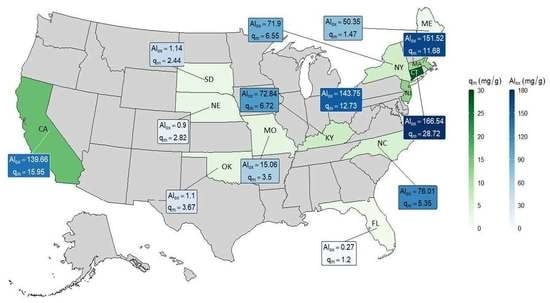
| Alox (mg/g) | 143.75 | 15.06 | 1.14 | 71.9 | 0.27 | 1.1 | 0.9 | 72.84 | 50.35 | 139.66 | 76.01 | 151.52 | 166.54 | ||
| Feox (mg/g) | 6.59 | 5.67 | 0.99 | 6.68 | 0.48 | 9.82 | 1.34 | 3.87 | 7.34 | 2.49 | 21.74 | 22.18 | 7.46 | ||
| Pox (mg/g) | 1.96 | 0.49 | 0.3 | 0.45 | 0.01 | 0.12 | 0.08 | 0.66 | 0.14 | 1.17 | 0.35 | 0.21 | 0.74 | ||
| Total P (mg/g) | 2.76 | 0.7 | 0.4 | 0.9 | 0.04 | 0.15 | 0.96 | 1.92 | 0.51 | 2.5 | 2.4 | 0.8 | 0.86 | ||
| PSI (%) | 1.16 | 2.4 | 16.12 | 0.52 | 2.59 | 1.77 | 4.31 | 0.77 | 0.23 | 0.73 | 0.35 | 0.11 | 0.38 | ||
| Sample (State) | NJ | MO | SD | NY | FL | OK | NE | KY | ME | CA | NC | MA | CT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qm (mg/g) | 12.73 | 3.50 | 2.44 | 6.55 | 1.20 | 3.67 | 2.82 | 6.72 | 1.47 | 15.95 | 5.35 | 11.68 | 28.72 |
| qm (mg/g) | Alox (mg/g) | Feox (mg/g) | Pox (mg/g) | Total Al (mg/g) | Total Fe (mg/g) | Total P (mg/g) | PSI | pH | OM (%) | C (%) | N (%) | S (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qm (mg/g) | 1.0000 | ||||||||||||
| Alox (mg/g) | 0.8521 | 1.0000 | |||||||||||
| Feox (mg/g) | 0.1404 | 0.3998 | 1.0000 | ||||||||||
| Pox (mg/g) | 0.5363 | 0.6395 | −0.1123 | 1.0000 | |||||||||
| Total Al (mg/g) | 0.6022 | 0.8511 | 0.5893 | 0.4452 | 1.0000 | ||||||||
| Total Fe (mg/g) | −0.3701 | −0.4834 | 0.2266 | −0.4191 | −0.3781 | 1.0000 | |||||||
| Total P (mg/g) | 0.3520 | 0.5846 | 0.1840 | 0.7698 | 0.6238 | −0.1739 | 1.0000 | ||||||
| PSI | −0.3243 | −0.4913 | −0.4111 | −0.1887 | −0.5782 | 0.0415 | −0.3159 | 1.0000 | |||||
| pH | −0.4045 | −0.6521 | −0.3931 | −0.4807 | −0.8257 | 0.3027 | −0.6512 | 0.6881 | 1.0000 | ||||
| OM (%) | 0.2422 | 0.5104 | 0.0074 | 0.8189 | 0.4975 | −0.3804 | 0.6754 | −0.1718 | −0.5628 | 1.0000 | |||
| C (%) | −0.0227 | −0.1063 | −0.4680 | −0.1752 | −0.1380 | −0.2008 | −0.3125 | 0.0898 | 0.1443 | 0.1299 | 1.0000 | ||
| N (%) | 0.6494 | 0.8293 | 0.2404 | 0.5396 | 0.8986 | −0.5541 | 0.6100 | −0.5212 | −0.7085 | 0.5108 | 0.0710 | 1.0000 | |
| S (%) | 0.0969 | 0.3421 | −0.1883 | 0.4676 | 0.4749 | −0.4627 | 0.3908 | −0.3309 | −0.5141 | 0.7141 | 0.4462 | 0.6653 | 1.0000 |
| Source | DF | Sum of Squares | Mean Square | F Ratio |
| Model | 1 | 526.369 | 526.369 | 29.167 |
| Error | 11 | 198.516 | 18.047 | Prob > F |
| C. Total | 12 | 724.886 | 0.0002 * | |
| Term | Estimate | Std Error | t Ratio | Prob > |t| |
| Intercept | 0.790 | 1.768 | 0.45 | 0.664 |
| Alox (mg/g) | 0.104 | 0.019 | 5.40 | 0.0002 * |
| RSquare Adj | 0.701 |
| Source | DF | Sum of Squares | Mean Square | F Ratio | |
| Model | 3 | 631.932 | 210.644 | 20.395 | |
| Error | 9 | 92.954 | 10.328 | Prob > F | |
| C. Total | 12 | 724.886 | 0.0002 * | ||
| Term | Estimate | Std Error | t Ratio | Prob > |t| | VIF |
| Intercept | 3.912 | 1.658 | 2.36 | 0.0427 * | |
| Alox (mg/g) | 0.137 | 0.018 | 7.66 | <0.0001 * | 1.5164 |
| Feox (mg/g) | −0.416 | 0.155 | −2.68 | 0.0250 * | 1.3881 |
| S (%) | −0.645 | 0.246 | −2.62 | 0.0278 * | 1.3208 |
| RSquare Adj | 0.829 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmati, R.; Sidhu, V.; Nunez, R.; Datta, R.; Sarkar, D. Correlation of Phosphorus Adsorption with Chemical Properties of Aluminum-Based Drinking Water Treatment Residuals Collected from Various Parts of the United States. Molecules 2022, 27, 7194. https://doi.org/10.3390/molecules27217194
Rahmati R, Sidhu V, Nunez R, Datta R, Sarkar D. Correlation of Phosphorus Adsorption with Chemical Properties of Aluminum-Based Drinking Water Treatment Residuals Collected from Various Parts of the United States. Molecules. 2022; 27(21):7194. https://doi.org/10.3390/molecules27217194
Chicago/Turabian StyleRahmati, Roxana, Virinder Sidhu, Rosita Nunez, Rupali Datta, and Dibyendu Sarkar. 2022. "Correlation of Phosphorus Adsorption with Chemical Properties of Aluminum-Based Drinking Water Treatment Residuals Collected from Various Parts of the United States" Molecules 27, no. 21: 7194. https://doi.org/10.3390/molecules27217194
APA StyleRahmati, R., Sidhu, V., Nunez, R., Datta, R., & Sarkar, D. (2022). Correlation of Phosphorus Adsorption with Chemical Properties of Aluminum-Based Drinking Water Treatment Residuals Collected from Various Parts of the United States. Molecules, 27(21), 7194. https://doi.org/10.3390/molecules27217194