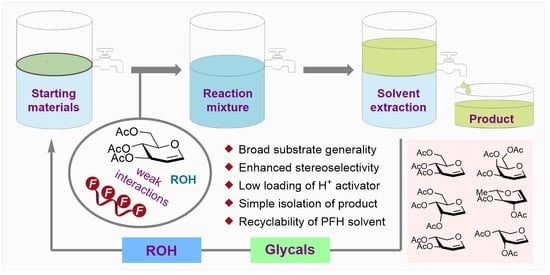
Acid Catalyzed Stereocontrolled Ferrier-Type Glycosylation Assisted by Perfluorinated Solvent
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Reaction Conditions
2.2. Substrate Scope
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Polkowski, K.; Popiołkiewicz, J.; Krzeczyński, P.; Ramza, J.; Pucko, W.; Zegrocka-Stendel, O.; Boryski, J.; Skierski, J.S.; Ma-zurek, A.P.; Grynkiewicz, G. Cytostatic and Cytotoxic Activity of Synthetic Genistein Glycosides Against Human Cancer Cell Lines. Cancer Lett. 2004, 203, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Popiołkiewicz, J.; Polkowski, K.; Skierski, J.S.; Mazurek, A.P. In Vitro Toxicity Evaluation in the Development of New Anticancer Drugs–Genistein Glycosides. Cancer Lett. 2005, 229, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Babu, R.S.; Zhou, M.; O’Doherty, G.A. De Novo Synthesis of Oligosaccharides Using a Palladium-Catalyzed Glycosylation Reaction. J. Am. Chem. Soc. 2004, 126, 3428–3429. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Veitschegger, A.M.; Nie, S.; Relitti, N.; MacRae, A.J.; Jurica, M.S. Enantioselective Synthesis of Thailanstatin A Methyl Ester and Evaluation of in Vitro Splicing Inhibition. J. Org. Chem. 2018, 83, 5187–5198. [Google Scholar] [CrossRef] [PubMed]
- Ferrier, R.J. Unsaturated Carbohydrates. Part II. Three Reactions Leading to Unsaturated Glycopyranosides. J. Chem. Soc. 1964, 5443–5449. [Google Scholar] [CrossRef]
- Ferrier, R.J.; Prasad, N. Unsaturated Carbohydrates. Part XI. Isomerisation and Dimerisation of Tri-O-acetyl-D-glucal. J. Chem. Soc. C 1969, 581–586. [Google Scholar] [CrossRef]
- Gómez, A.M.; Lobo, F.; Uriel, C.; López, J.C. Recent Developments in the Ferrier Rearrangement. Eur. J. Org. Chem. 2013, 32, 7221–7262. [Google Scholar] [CrossRef] [Green Version]
- Gómez, A.M.; Lobo, F.; Miranda, S.; López, J.C. A Survey of Recent Synthetic Applications of 2,3-Dideoxy-Hex-2-enopyranosides. Molecules 2015, 20, 8357–8394. [Google Scholar] [CrossRef] [Green Version]
- Jiang, N.; Wu, Z.; Dong, Y.; Xu, X.; Liu, X.; Zhang, J. Progress in the Synthesis of 2,3-Unsaturated Glycosides. Curr. Org. Chem. 2020, 24, 184–199. [Google Scholar] [CrossRef]
- Bennett, C.S.; Galan, M.C. Methods for 2-Deoxyglycoside Synthesis. Chem. Rev. 2018, 118, 7931–7985. [Google Scholar] [CrossRef]
- McKay, M.J.; Nguyen, H.M. Recent Advances in Transition Metal-Catalyzed Glycosylation. ACS Catal. 2012, 2, 1563–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, E.B. Transition Metal Catalyzed Glycosylation Reactions—An Overview. Org. Biomol. Chem. 2020, 18, 9160–9180. [Google Scholar] [CrossRef] [PubMed]
- Gómez, A.M.; Valverde, S.; Fraser-Reid, B. A Route to Unsaturated Spiroketals from Phenylthio Hex-2-enopyranosides via Sequential alkylation, Allylic Rearrangement and Intramolecular Glycosidation. J. Chem. Soc. Chem. Commun. 1991, 1207–1208. [Google Scholar] [CrossRef]
- Rafiee, E.; Tangestaninejad, S.; Habibi, M.H.; Mirkhani, V. A Mild, Efficient and α-Selective Glycosidation by Using Potassium Dodecatungstocobaltate Trihydrate as Catalyst. Bioorg. Med. Chem. Lett. 2004, 14, 3611–3614. [Google Scholar] [CrossRef]
- Leng, W.-L.; Yao, H.; He, J.-X.; Liu, X.-W. Venturing beyond Donor-Controlled Glycosylation: New Perspectives toward Anomeric Selectivity. Acc. Chem. Res. 2018, 51, 628–639. [Google Scholar] [CrossRef]
- Alabugin, I.V.; Kuhn, L.; Medvedev, M.G.; Krivoshchapov, N.V.; Vil’, V.A.; Yaremenko, I.A.; Mehaffy, P.; Yarie, M.; Terent’ev, A.O.; Zolfigol, M.A. Stereoelectronic Power of Oxygen in Control of Chemical Reactivity: The Anomeric Effect is Not Alone. Chem. Soc. Rev. 2021, 50, 10253–10345. [Google Scholar] [CrossRef]
- Schuff, B.P.; Mercer, G.J.; Nguyen, H.M. Palladium-Catalyzed Stereoselective Formation of α-O-Glycosides. Org. Lett. 2007, 9, 3173–3176. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.-H.; Hoang, K.L.; He, M.J.; Tan, Y.-J.; Liu, X.-W. Reversing the Stereoselectivity of a Palladium-Catalyzed O-Glycosylation through an Inner-sphere or Outer-sphere Pathway. Angew. Chem. Int. Ed. 2015, 54, 604–607. [Google Scholar]
- Kim, H.; Men, H.; Lee, C. Stereoselective Palladium-Catalyzed O-Glycosylation Using Glycals. J. Am. Chem. Soc. 2004, 126, 1336–1337. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Zhang, S.; Leng, W.-L.; Leow, M.-L.; Xiang, S.; He, J.; Liao, H.; Hoang, K.L.M.; Liu, X.-W. Catalyst-Controlled Stereoselective O-Glycosylation: Pd(0) vs Pd(II). ACS Catal. 2017, 7, 5456–5460. [Google Scholar] [CrossRef]
- Comely, A.C.; Eelkema, R.; Minnaard, A.J.; Feringa, B.L. De Novo Asymmetric Bio- and Chemocatalytic Synthesis of Saccharides–Stereoselective Formal O-Glycoside Bond Formation Using Palladium Catalysis. J. Am. Chem. Soc. 2003, 125, 8714–8715. [Google Scholar] [CrossRef]
- Babu, R.S.; O’Doherty, G.A. A Palladium-Catalyzed Glycosylation Reaction: The de Novo Synthesis of Natural and Unnatural Glycosides. J. Am. Chem. Soc. 2003, 125, 12406–12407. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Lu, Z.; He, J.; Hoang, K.L.M.; Zeng, J.; Liu, X.-W. β-Type Glycosidic Bond Formation by Palladium-Catalyzed Decarboxylative Allylation. Chem. Eur. J. 2013, 19, 14047–14051. [Google Scholar] [CrossRef]
- Wang, Q.; Lai, M.; Luo, H.; Ren, K.; Wang, J.; Huang, N.; Deng, Z.; Zou, K.; Yao, H. Stereoselective O-Glycosylation of Glycals with Arylboronic Acids Using Air as the Oxygen Source. Org. Lett. 2022, 24, 1587–1592. [Google Scholar] [CrossRef]
- Loh, C.C.J. Exploiting Non-Covalent Interactions in Selective Carbohydrate Synthesis. Nat. Rev. Chem. 2021, 5, 792–815. [Google Scholar] [CrossRef]
- Betzemeier, B.; Knochel, P. Modern Solvents in Organic Synthesis: Perfluorinated Solvents–A Novel Reaction Medium in Organic Chemistry. Top. Curr. Chem. 1999, 206, 60–78. [Google Scholar]
- Berger, R.; Resnati, G.; Metrangolo, P.; Weberd, E.; Hulliger, J. Organic Fluorine Compounds: A Great Opportunity for Enhanced Materials Properties. Chem. Soc. Rev. 2011, 40, 3496–3508. [Google Scholar] [CrossRef] [PubMed]
- Klement, I.; Knochel, P. Selective Oxidation of Zinc Organometallics to Hydroperoxides Using Oxygen in Perfluorohexanes. Synlett 1995, 27, 1113–1114. [Google Scholar] [CrossRef]
- Brown, H.C.; Negishi, E. Bisborolane. Highly Elusive Bisboracyclane. J. Am. Chem. Soc. 1971, 93, 6682–6683. [Google Scholar] [CrossRef]
- Brown, H.C.; Midland, M.M.; Kabalka, G.W. Stoichiometrically Controlled Reaction of Organoboranes with Oxygen Under very Mild Conditions to Achieve Essentially Quantitative Conversion into Alcohols. J. Am. Chem. Soc. 1971, 93, 1024–1025. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Jang, D.O.; Jaszberenyi, J.C. An Improved Radical Chain Procedure for the Deoxygenation of Secondary and Primary Alcohols using Diphenylsilane as Hydrogen Atom Donor and Triethylborane-Air as Initiator. Tetrahedron Lett. 1990, 31, 4681–4684. [Google Scholar] [CrossRef]
- Klement, I.; Lütjens, H.; Knochel, P. Transition Metal Catalyzed Oxidations in Perfluorinated Solvents. Angew. Chem. Int. Ed. 1997, 36, 1454–1456. [Google Scholar] [CrossRef]
- Tada, N.; Cui, L.; Ishigami, T.; Ban, K.; Miura, T.; Uno, B.; Itoh, A. Facile Aerobic Photooxidative Oxylactonization of Oxocarboxylic Acids in Fluorous Solvents. Green Chem. 2012, 14, 3007–3009. [Google Scholar] [CrossRef]
- Karimi, M.; Sadeghi, S.; Mohebali, H.; Azarkhosh, Z.; Safarifard, V.; Mahjoub, A.; Heydari, A. Fluorinated Solvent-Assisted Photocatalytic Aerobic Oxidative Amidation of Alcohols via Visible-Light-Mediated HKUST-1/Cs-POMoW Catalysis. New J. Chem. 2021, 45, 14024–14035. [Google Scholar] [CrossRef]
- Horváth, I.T.; Rábi, J. Facile Catalyst Separation Without Water: Fluorous Biphases Hydroformylation of Olefins. Science 1994, 266, 72–75. [Google Scholar] [CrossRef]
- Maayan, G.; Fish, R.H.; Neumann, R. Polyfluorinated Quaternary Ammonium Salts of Polyoxometalate Anions: Fluorous Biphasic Oxidation Catalysis with and without Fluorous Solvents. Org. Lett. 2003, 5, 3547–3550. [Google Scholar] [CrossRef]
- O’Hagan, D. Understanding Organofluorine Chemistry. An Introduction to the C–F Bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef]
- Champagne, P.A.; Desroches, J.; Paquin, J.-F. Organic Fluorine as a Hydrogen-Bond Acceptor: Recent Examples and Applications. Synthesis 2015, 47, 306–322. [Google Scholar]
- Yu, J.-S.; Liu, Y.-L.; Tang, J.; Wang, X.; Zhou, J. Highly Efficient “On Water” Catalyst-Free Nucleophilic Addition Reactions Using Difluoroenoxysilanes: Dramatic Fluorine Effects. Angew. Chem. Int. Ed. 2014, 53, 9512–9516. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, W.; Liao, K.; Wang, X.; Zhou, J.; Ma, J. Catalytic Enantioselective Synthesis of Cyclopropanes Featuring Vicinal All-Carbon Quaternary Stereocenters with a CH2F Group; Study of Influence of C-F···H-N Interactions on Reactivity. Org. Chem. Front. 2018, 5, 2960–2968. [Google Scholar] [CrossRef]
- Myers, K.E.; Kumar, K. Fluorophobic Acceleration of Diels-Alder Reactions. J. Am. Chem. Soc. 2000, 122, 12025–12026. [Google Scholar] [CrossRef]
- Piscelli, B.A.; Sanders, W.; Yu, C.; Maharik, N.A.; Lebl, T.; Cormanich, R.A.; O’Hagan, D. Fluorine-Induced Pseudo-Anomeric Effects in Methoxycyclohexanes through Electrostatic 1,3-Diaxial Interactions. Chem. Eur. J. 2020, 26, 11989–11994. [Google Scholar] [CrossRef] [PubMed]
- Misbahi, K.; Lardic, M.; Ferrières, V.; Noiret, N.; Kerbal, A.; Plusquellec, D. Unexpected fluorous solvent effect on oxidation of 1-thioglycosides. Tetrahedron Asymmetry 2001, 12, 2389–2393. [Google Scholar] [CrossRef]
- Oikawa, M.; Tanak, T.; Fukud, N.; Kusumoto, S. One-Pot Preparation and Activation of Glycosyl Trichloroacetimidates: Operationally Simple Glycosylation Induced by Combined Use of Solid-Supported, Reactivity-Opposing Reagents. Tetrahedron Lett. 2004, 45, 4039–4042. [Google Scholar] [CrossRef]
- Farrán, A.; Cai, C.; Sandoval, M.; Xu, Y.; Liu, J.; Hernáiz, M.J.; Linhardt, R.J. Green Solvents in Carbohydrate Chemistry: From Raw Materials to Fine Chemicals. Chem. Rev. 2015, 115, 6811–6853. [Google Scholar] [CrossRef]
- Di Salvo, A.; David, M.; Crousse, B.; Bonnet-Delpon, D. Self-Promoted Nucleophilic Addition of Hexafluoro-2-propanol to Vinyl Ethers. Adv. Synth. Catal. 2006, 348, 118–124. [Google Scholar] [CrossRef]
- Nakano, H.; Kitazume, T. Organic Reactions without an Organic Medium–Utilization of Perfluorotriethylamine as a Reaction Medium. Green Chem. 1999, 1, 21–22. [Google Scholar] [CrossRef]
- Gorityala, B.K.; Lorpitthaya, R.; Bai, Y.; Liu, X.-W. ZnCl2/Alumina Impregnation Catalyzed Ferrier Rearrangement: An Expedient Synthesis of Pseudoglycosides. Tetrahedron 2009, 65, 29–30. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, H.; Shan, J.; Li, J.; Yang, G.; Chen, X.; Xin, K.; Zhang, J.; Tang, J. FeCl3·6H2O/C: An Efficient and Recyclable Catalyst for the Synthesis of 2,3-Unsaturated O- and S-Glycosides. J. Carbohydr. Chem. 2014, 33, 313–325. [Google Scholar] [CrossRef]
- Santra, A.; Guchhait, G.; Misra, A. Nitrosyl Tetrafluoroborate Catalyzed Preparation of 2,3-Unsaturated Glycosides and 2-Deoxyglycosides of Hindered Alcohols, Thiols, and Sulfonamides. Synlett 2013, 24, 581–586. [Google Scholar] [CrossRef]
- Ruan, Z.; Dabideen, D.; Blumenstein, M.; Mootoo, D.R.A. Modular Synthesis of the Bis-Tetrahydrofuran Core of Rolliniastatin from Pyranoside Precursors. Tetrahedron 2000, 56, 9203–9211. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Pandey, S.K. Ceric (IV) Ammonium Nitrate-Catalyzed Glycosidation of Glycals: A Facile Synthesis of 2,3-Unsaturated Glycosides. New J. Chem. 2001, 25, 538–540. [Google Scholar] [CrossRef]
- Srinivas, B.; Reddy, T.R.; Radha Krishna, P.; Kashyap, S. Copper (II) Triflate as a Mild and Efficient Catalyst for Ferrier Glycosylation: Synthesis of 2,3-Unsaturated O-Glycosides. Synlett 2014, 25, 1325–1330. [Google Scholar] [CrossRef]
- Saeeng, R.; Siripru, O.; Sirion, U. IBr-Catalyzed O-Glycosylation of D-Glucals: Facile Synthesis of 2,3-Unsaturated-O-Glycosides. Heterocycles 2015, 91, 849–861. [Google Scholar]
- Bound, D.J.; Bettadaiah, B.K.; Srinivas, P. ZnBr2-Catalyzed and Microwave-Assisted Synthesis of 2,3-Unsaturated Glucosides of Hindered Phenols and Alcohols. Synth. Commun. 2014, 44, 2565–2576. [Google Scholar] [CrossRef]
- Frappa, I.; Sinou, D. An Easy and Efficient Preparation of Aryl α-O-Δ2−Glycosides. Synth. Commun. 1995, 25, 2941–2951. [Google Scholar] [CrossRef]
- Sun, G.; Qiu, S.; Ding, Z.; Chen, H.; Zhou, J.; Wang, Z.; Zhang, J. Magnetic Core-Shell Fe3O4@C-SO3H as an Efficient and Renewable ‘Green Catalyst’ for the Synthesis of O-2,3-Unsaturated Glycopyranosides. Synlett 2017, 28, 347–352. [Google Scholar]
- Babu, B.S.; Balasubramanian, K.K. Indium Trichloride Catalyzed Glycosidation. An Expeditious Synthesis of 2,3-Unsaturated Glycopyranosides. Tetrahedron Lett. 2000, 41, 1271–1274. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Murthy, C.V.S.R.; Kumar, G.M. Scandium Triflate Catalyzed Ferrier Rearrangement: An Efficient Synthesis of 2,3-Unsaturated Glycopyranosides. Synlett 2000, S11210, 1450–1451. [Google Scholar] [CrossRef]
- Stevanović, D.; Pejović, A.; Damljanović, I.; Vukićević, R.D.; Vukić ević, M.; Bogdanovic, G.A. Anodic Generation of a Zirconium Catalyst for Ferrier Rearrangement and Hetero Michael Addition. Tetrahedron Lett. 2012, 53, 6257–6260. [Google Scholar] [CrossRef]
- Stevanović, D.; Pejović, A.; Damljanović, I.; Minić, A.; Bogdanović, G.A.; Vukićević, M.; Radulović, N.S.; Vukićević, R.D. Ferrier Rearrangement Promoted by an Electrochemically Generated Zirconium Catalyst. Carbohydr. Res. 2015, 407, 111–121. [Google Scholar] [CrossRef]
- Bhagavathy, S.; Ajay, K.B.; Kalpattu, K.B. Microwave-induced, Montmorillonite K10-Catalyzed Ferrier Rearrangement of Tri-O-Acetyl-D-Galactal: Mild, Eco-Friendly, Rapid Glycosidation with Allylic Rearrangement. Tetrahedron Lett. 2002, 43, 6795–6798. [Google Scholar]
- Grynkiewicz, G.; Priebe, W.; Zamojski, A. Synthesis of Alkyl 4, 6-di-O-Acetryl-2,3-Dideoxy-a-D-Three-hex-2-Enopyranosides from 3,4,6-tri-O-Acetyl-1,5-Anhydro-2-Droxy D-lyxo-hex-1-enitol(3,4,6-tri-O-acetyl-e-galactal). Carbohydr. Res. 1979, 68, 33–41. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, D.D. Sm(OTf)3 as a Highly Effificient Catalyst for the Synthesis of 2,3-Unsaturated O- and S-Pyranosides from Glycals and the Temperature-Dependent Formation of 4-O-Acetyl-6-Deoxy-2,3-Unsaturated S-Pyranosides and 4-O-Acetyl-6-Deoxy-3-Alkylthio Glycals. Tetrahedron 2014, 70, 8505–8510. [Google Scholar] [CrossRef]
- Tatina, M.B.; Mengxin, X.; Peilin, R.; Judeh, Z.M.A. Robust Perfluorophenylboronic Acid-Catalyzed Stereoselective Synthesis of 2,3-Unsaturated O-, C-, N- and S-linked Glycosides. Beilstein J. Org. Chem. 2019, 15, 1275–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.H.; Jacobs, P.B.; Elliott, R.L.; Curran, D.P. A Gentral- Synthetic Approach to S113 Optically Active Iridoid Aglpoaes. The Total Synthesis of Beta-Ethyl Descarbomethoxyverbenalol, Ethyl Catalpot, and (-)-Specionin. Tetrahedron 2014, 44, 3079–3092. [Google Scholar]
- Bartlett, M.J.; Peter, T.; Northcote, P.T.; Lein, M.; Harvey, J.E. 13C NMR Analysis of 3,6-Dihydro-2H-pyrans: Assignment of Remote Stereochemistry Using Axial Shielding Effects. J. Org. Chem. 2014, 79, 5521–5532. [Google Scholar] [CrossRef]
- Khan, A.T.; Sidick Basha, R.S.; Lal, M. Bromodimethylsulfonium Bromide (BDMS) Catalyzed Synthesis of 2,3-Unsaturated-O-Glycosides via Ferrier Rearrangement. Arkivoc 2012, 2013, 201–212. [Google Scholar] [CrossRef]




 | ||||||
|---|---|---|---|---|---|---|
| Entry a | Additive | Catalyst b | Solvent | Temp. (°C) | Yield (%) c | Stereoselectivity (α:β) |
| 1 | TFE | - | Ethanol | 100 | 45 | >20:1 |
| 2 | PFD | - | Ethanol | 100 | 55 | >20:1 |
| 3 | PFH | - | Ethanol | 100 | 60 | >20:1 |
| 4 | PFTEA | - | Ethanol | 100 | 15 | >20:1 |
| 5 | - | - | Ethanol | 100 | <10 | 5:1 |
| 6 | - | - | PFH | 100 | trace | - |
| 7 | - | resin-H+ | PFH | 100 | 96 | >20:1 |
| 8 | - | resin-H+ | CH2Cl2 | 100 | 16 | 1.5:1 |
| 9 | - | resin-H+ | Ethanol | 100 | 85 | 7:1 |
| 10 | PFH | resin-H+ | Hexane | 100 | - | - |
| 11 | PFH | resin-H+ | Toluene | 100 | trace | - |
| 12 | PFH | resin-H+ | DCE | 100 | trace | - |
| 13 | PFH | resin-H+ | DMF | 100 | - | - |
| 14 | - | resin-H+ | PFH | 80 | 55 | >20:1 |
| 15 d | - | resin-H+ | PFH | 80 | 95 | >20:1 |
| 16 | - | resin-H+ | PFH | 60 | trace | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Li, Y.; Xiang, S.; Zuo, M.; Sun, Y.; Jiang, X.; Jiao, R.; Wang, Y.; Fu, Y. Acid Catalyzed Stereocontrolled Ferrier-Type Glycosylation Assisted by Perfluorinated Solvent. Molecules 2022, 27, 7234. https://doi.org/10.3390/molecules27217234
Lu Z, Li Y, Xiang S, Zuo M, Sun Y, Jiang X, Jiao R, Wang Y, Fu Y. Acid Catalyzed Stereocontrolled Ferrier-Type Glycosylation Assisted by Perfluorinated Solvent. Molecules. 2022; 27(21):7234. https://doi.org/10.3390/molecules27217234
Chicago/Turabian StyleLu, Zhiqiang, Yanzhi Li, Shaohua Xiang, Mengke Zuo, Yangxing Sun, Xingxing Jiang, Rongkai Jiao, Yinghong Wang, and Yuqin Fu. 2022. "Acid Catalyzed Stereocontrolled Ferrier-Type Glycosylation Assisted by Perfluorinated Solvent" Molecules 27, no. 21: 7234. https://doi.org/10.3390/molecules27217234
APA StyleLu, Z., Li, Y., Xiang, S., Zuo, M., Sun, Y., Jiang, X., Jiao, R., Wang, Y., & Fu, Y. (2022). Acid Catalyzed Stereocontrolled Ferrier-Type Glycosylation Assisted by Perfluorinated Solvent. Molecules, 27(21), 7234. https://doi.org/10.3390/molecules27217234