An Overview on the Synthesis of Fused Pyridocoumarins with Biological Interest
Abstract
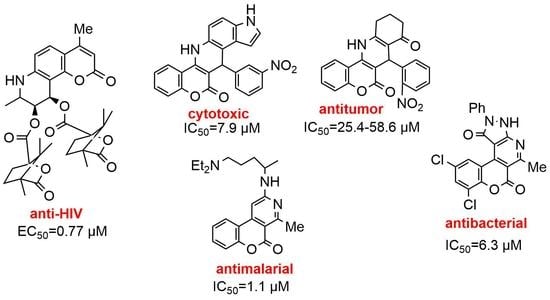
:1. Introduction
2. Synthetic Strategies for the Preparation of Fused Pyridocoumarins
2.1. Pyridine-Ring Formation
2.1.1. Synthesis from Aminocoumarins
Skraup Reaction
Reaction with α,β-Unsaturated Carbonyl Compounds (Skraup–Doebner–von Miller Reaction)
Povarov Reaction
Friedlander Reaction
From Propargylaminocoumarins
Multi Component Reactions (MCR) of Aminocoumarin
Metal-Catalyzed Reactions of Aminocoumarin Derivatives
2.1.2. Synthesis from Hydroxycoumarins
Multi Component Reactions (MCR) of Hydroxycoumarins
Synthesis with Krohnke’s-Type Reaction
2.1.3. Synthesis from Various Coumarin Derivatives
2.2. Pyranone Ring Formation
2.2.1. Synthesis from Pyridine or Piperidine Derivatives
2.2.2. Synthesis from Phenol or Salicylaldehyde Derivatives
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdelmohsen, U.R.; Albohy, A.; Abdulrazik, B.S.; Bayoumi, S.A.L.; Malak, L.G.; Khallaf, I.S.A.; Bringmann, G.; Farag, S.F. Natural coumarins as potential anti-SARS-CoV-2 agents supported by docking analysis. RSC Adv. 2021, 11, 16970–16979. [Google Scholar] [CrossRef] [PubMed]
- Stringlis, I.A.; de Jonge, R.; Pieterse, C.M.J. The age of coumarins in plant-microbe interactions. Plant Cell Physiol. 2019, 60, 1405–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voges, M.J.E.E.E.; Baic, Y.; Schulze-Lefert, P.; Sattely, E.S. Plant-derived coumarins shape the composition of an Arabidopsis synthetic root microbiome. Proc. Natl. Acad. Sci. USA 2019, 116, 12558–12565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yord, E.G. Coumarins—An Important Class of Phytochemicals. In Phytochemicals: Isolation, Characterisation and Role in Human Health; Rao, V.A., Rao, L.G., Eds.; IntechOpen: Rijeka, Croatia, 2015; Chapter 5. [Google Scholar]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed. Res. Intern. 2013, 2013, 963248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awe, S.; Mikolasch, A.; Schauer, F. Formation of coumarines during the degradation of alkyl substituted aromatic oil components by the yeast Trichosporon asahii. Appl. Microbiol. Biotechnol. 2009, 84, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Su, X.-H.; Zhang, M.-L.; Li, L.-G.; Huo, C.-H.; Gu, Y.-C.; Shi, Q.-W. Chemical Constituents of the Plants of the Genus Calophyllum. Chem. Biodivers. 2008, 5, 2579–2608. [Google Scholar] [CrossRef] [PubMed]
- Murray, D.H.; Mendez, J.; Brown, S.A. The Natural Coumarins: Occurrence, Chemistry and Biochemistry; John Wiley & Sons: New York, NY, USA, 1982. [Google Scholar]
- O’Kennedy, R.; Thornes, R.D. Coumarins: Biology, Applications and Mode of Action; John Wiley & Sons: Chichester, UK, 1997. [Google Scholar]
- Kontogiorgis, C.; Detsi, A.; Hadjipavlou-Litina, D. Coumarin-based drugs: A patent review (2008–present). Expert Opin. Ther. Pat. 2012, 22, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Medina, F.G.; Marrero, J.G.; Macías-Alonso, M.; González, M.C.; CórdovaGuerrero, I.; Teissier García, A.G.; Osegueda-Robles, S. Coumarin heterocyclic derivatives: Chemical synthesis and biological activity. Nat. Prod. Rep. 2015, 32, 1472–1507. [Google Scholar] [CrossRef] [PubMed]
- Borah, B.; Dwivedi, K.D.; Kumar, B.; Chowhan, L.R. Recent advances in the microwave- and ultrasound-assisted green synthesis of coumarin-heterocycles. Arab. J. Chem. 2022, 15, 103654. [Google Scholar] [CrossRef]
- O’Reilly, R.; Aggeler, P.M. Studies on Coumarin Anticoagulant Drugs. Initiation of Warfarin Therapy Without a Loading Dose. Circulation 1968, 38, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Lowenthal, J.; Birnbaum, H. Vitamin K and coumarin anticoagulants: Dependence of anticoagulant effect on inhibition of vitamin K transport. Science 1969, 164, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Fisher, P.; Campbell, K.J.; Howald, G.R.; Warburton, B. Anticoagulant Rodenticides, Islands, and Animal Welfare Accountancy. Animals 2019, 9, 919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fylaktakidou, K.C.; Hadjipavlou-Litina, D.J.; Litinas, K.E.; Nicolaides, D.N. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr. Pharm. Des. 2004, 10, 3813–3833. [Google Scholar] [CrossRef] [PubMed]
- Kontogiorgis, C.; Hadjipavlou-Litina, D.J. Synthesis and antiinflammatory activity of coumarin derivatives. J. Med. Chem. 2005, 48, 6400–6408. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Suzuki, M.; Xie, L.; Morris-Natschke, S.L.; Lee, K.H. Recent progress in the development of coumarin derivatives as potent anti-HIV agents. Res. Rev. 2003, 23, 322–345. [Google Scholar] [CrossRef]
- Wang, L.; Ma., T.; Liu, G. Recent progress in calophyllum coumarins as potent anti-HIV agents. In Medicinal Chemistry of Bioactive Natural Products; Liang, X.T., Fang, W.S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2006; p. 326. [Google Scholar]
- Xu, Z.; Chen, Q.; Zhang, Y.; Liang, C. Coumarin-based derivatives with potential anti-HIV activity. Fitoterapia 2021, 150, 104863. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hu, D.; Wu, Z.; Wei, C.; Wu, S.; Song, B. Coumarin Derivatives Containing Sulfonamide and Dithioacetal Moieties: Design, Synthesis, Antiviral Activity, and Mechanism. J. Agric. Food Chem. 2022, 70, 5773–5783. [Google Scholar] [CrossRef]
- Lacy, A.; O’Kennedy, R. Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr. Pharm. Des. 2004, 10, 3797–3811. [Google Scholar] [CrossRef] [Green Version]
- Promsuwan, P.; Yenjai, C. Synthesis and Cytotoxicity of Coumarin Derivatives and Nordentatin. Asian J. Chem. 2013, 25, 3629–3632. [Google Scholar] [CrossRef]
- Thakur, A.; Singla, R.; Jaitak, V. Coumarins as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2015, 101, 476–495. [Google Scholar] [CrossRef]
- Rubab, L.; Afroz, S.; Ahmad, S.; Hussain, S.; Nawaz, I.; Irfan, A.; Batool, F.; Kotwica-Mojzych, K.; Mojzych, M. An Update on Synthesis of Coumarin Sulfonamides as Enzyme Inhibitors and Anticancer Agents. Molecules 2022, 27, 1604. [Google Scholar] [CrossRef] [PubMed]
- May, J.M.; Owens, T.W.; Mandler, M.D.; Simpson, B.W.; Lazarus, M.B.; Sherman, D.J.; Davis, R.M.; Okuda, S.; Massefski, W.; Ruiz, N.; et al. The Antibiotic Novobiocin Binds and Activates the ATPase That Powers Lipopolysaccharide Transport. J. Am. Chem. Soc. 2017, 139, 17221–17224. [Google Scholar] [CrossRef] [Green Version]
- Madeiro, S.A.L.; Borges, N.H.P.B.; Souto, A.L.; de Figueiredo, P.T.R.; Siqueira-Junior, J.P.; Tavares, J.F. Modulation of the antibiotic activity against multidrug resistant strains of coumarins isolated from Rutaceae species. Microb. Pathogen. 2017, 104, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Liu, Y.; Guo, R.; Tang, Y.; Shi, Z.; Zhang, M.; Wu, W.; Chen, Y.; Hou, K. An in-vitro coumarin antibiotic combination treatment of Pseudomonas aeruginosa biofilms. Nat. Prod. Commun. 2021, 16, 1–7. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; Spadaro, V.; Bruno, M.; Maggio, A.; Faraone, N.; Rosselli, S. Antimicrobial and Antioxidant Activities of Coumarins from the Roots of Ferulago campestris (Apiaceae). Molecules 2009, 14, 939–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domínguez-Mendoza, E.A.; Cornejo-Garrido, J.; Burgueño-Tapia, E.; Ordaz-Pichardo, C. Antidiabetic effect, antioxidant activity, and toxicity of 3′,4′-Di-O-acetyl-cis-khellactone in Streptozotocin-induced diabetic rats. Bioorg. Med. Chem. Lett. 2016, 26, 4086–4091. [Google Scholar] [CrossRef] [PubMed]
- Alshibl, H.M.; Al-Abdullah, E.S.; Haiba, M.E.; Alkahtani, H.M.; Awad, G.E.A.; Mahmoud, A.H.; Ibrahim, B.M.M.; Bari, A.; Villinger, A. Synthesis and Evaluation of New Coumarin Derivatives as Antioxidant, Antimicrobial, and Anti-Inflammatory Agents. Molecules 2020, 25, 3251. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.; Kavimani, S.; Kadalmani, B.; Ahmed, A.B.A.; Akbarsha, M.A.; Rao, M.V. Antidiabetic activity of leaf and callus extracts of Aegle marmelos in rabbit. Sci. Asia 2008, 34, 317–321. [Google Scholar] [CrossRef]
- Li, H.; Yao, Y.; Li, L. Coumarins as potential antidiabetic agents. J. Pharm. Pharmacol. 2017, 69, 1253–1264. [Google Scholar] [CrossRef] [Green Version]
- Ojala, T.; Remes, S.; Haansuu, P.; Vuorela, H.; Hiltunen, R.; Haatela, K.; Vuorela, P. Antimicrobial activity of some coumarin containing herbal plants growing in Finland. J. Ethnopharmacol. 2000, 73, 299–305. [Google Scholar] [CrossRef]
- Giri, R.R.; Lad, H.B.; Bhila, V.G.; Patel, C.V.; Brahmbhatt, D.I. Modified pyridine-substituted coumarins: A new class of antimicrobial and antitubercular agents. Synth. Commun. 2015, 45, 363–375. [Google Scholar] [CrossRef]
- Al-Majedy, Y.K.; Kadhum, A.A.H.; Al-Amiery, A.A.; Mohamad, A.B. Coumarins: The Antimicrobial. Rev. Pharm. 2017, 8, 62–70. [Google Scholar] [CrossRef]
- Hu, Y.-Q.; Xu, Z.; Zhang, S.; Wu, X.; Ding, J.-W.; Lv, Z.S.; Feng, L.S. Recent developments of coumarin-containing derivatives and their anti-tubercular activity. Eur. J. Med. Chem. 2017, 136, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.S.; Kongot, M.; Kumar, A. Coumarin hybrid derivatives as promising leads to treat tuberculosis: Recent developments and critical aspects of structural design to exhibit anti-tubercular activity. Tuberculosis 2021, 127, 102050. [Google Scholar] [CrossRef] [PubMed]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A Natural, Privileged and Versatile Scaffold for Bioactive Compounds. Molecules 2018, 25, 250. [Google Scholar] [CrossRef] [Green Version]
- Vlachou, E.-E.N.; Litinas, K.E. An overview on pyranocoumarins: Synthesis and biological activities. Curr. Org. Chem. 2019, 23, 2679–2721. [Google Scholar] [CrossRef]
- El-Sawy, E.R.; Abdelwahab, A.B.; Kirsch, G. Synthetic Routes to Coumarin (Benzopyrone)-Fused Five-Membered Aromatic Heterocycles Built on the α-Pyrone Moiety. Part 1: Five-Membered Aromatic Rings with One Heteroatom. Molecules 2021, 26, 483. [Google Scholar] [CrossRef]
- Salehian, F.; Nadri, H.; Jalili-Baleh, L.; Youseftabar-Miri, L.; Bukhari, S.N.A.; Foroumadi, A.; Küçükkilinç, T.T.; Sharifzadeh, M.; Khoobi, M. A review: Biologically active 3,4-heterocycle-fused coumarins. Eur. J. Med. Chem. 2021, 212, 113034. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.A.; Lad, H.B.; Pandya, K.R.; Patel, C.V.; Brahmbhatt, D.I. Synthesis of a new series of 2-(2-oxo-2H-chromen-3-yl)-5H-chromeno[4,3-b]pyridin-5-ones by two facile methods and evaluation of their antimicrobial activity. Med. Chem. Res. 2013, 22, 4745–4754. [Google Scholar] [CrossRef]
- Patel, M.A.; Bhila, V.G.; Patel, N.H.; Patel, A.K.; Brahmbhatt, D.I. Brahmbhatt Synthesis, characterization and biological evaluation of some pyridine and quinoline fused chromenone derivatives. Med. Chem. Res. 2012, 21, 4381–4388. [Google Scholar] [CrossRef]
- Dawane, B.S.; Konda, S.G.; Bodade, R.G.; Bhosale, R.B. An efficient one-pot synthesis of some new 2,4-diaryl pyrido[3,2-c]coumarins as potent antimicrobial agents. J. Heterocycl. Chem. 2010, 47, 237–241. [Google Scholar] [CrossRef]
- Goswami, L.; Gogoi, S.; Gogoi, J.; Baruah, R.K.; Boruah, R.; Gogoi, P. Facile Diversity-Oriented Synthesis of Polycyclic Pyridines and Their Cytotoxicity Effects in Human Cancer Cell Lines. ACS Comb. Sci. 2016, 18, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Hamama, W.S.; Ibrahim, M.E.; Metwalli, A.E.; Zoorob, H.H. New synthetic approach to coumarino[4,3-b]pyridine systems and potential cytotoxic evaluation. Med. Chem. Res. 2014, 23, 2615–2621. [Google Scholar] [CrossRef]
- Mulakayala, N.; Rambabu, D.; Raja, M.R.; Chaitanya, M.; Kumar, C.S.; Kalle, A.M.; Krishna, G.R.; Reddy, C.M.; Rao, M.V.B.; Pal, M. Ultrasound mediated catalyst free synthesis of 6H-1-benzopyrano[4,3-b]quinolin-6-ones leading to novel quinoline derivatives: Their evaluation as potential anti-cancer agents. Bioorg. Med. Chem. 2012, 20, 759–768. [Google Scholar] [CrossRef]
- Hosni, H.M.; Abdulla, M.M. Anti-inflammatory and analgesic activities of some newly synthesized pyridinedicarbonitrile and benzopyranopyridine derivatives. Acta Pharm. 2008, 58, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Görlitzer, K.; Enge, C.; Jones, P.G.; Jomaa, H.; Wiesner, J. Benzo[c][2,7]naphthyridine-2-yl-, 5-yl- and 2,5-diyl novaldiamines--synthesis and investigation of anti-malarial activity. Pharmazie 2006, 61, 975–980. [Google Scholar] [PubMed]
- Heber, D. Reaktionen an Heterocyclen mit 2-Acyl-2-propenon-Teilstruktur, 3. Mitt. Pyrido[3,2-c]cumarine aus 3-substituierten 1-Benzopyranen und Enaminen. Arch. Pharm. 1987, 320, 402–406. [Google Scholar] [CrossRef]
- Adib, M.; Peytam, F.; Rahmanian-Jazi, M.; Mohammadi-Khanaposhtani, M.; Mahernia, S.; Bijanzadeh, H.R.; Jahani, M.; Imanparast, S.; Faramarzi, M.A.; Mahdavig, M.; et al. Design, synthesis and in vitro α-glucosidase inhibition of novel coumarin-pyridines as potent antidiabetic agents. New J. Chem. 2018, 42, 7268–17278. [Google Scholar] [CrossRef]
- Ahn, S.; Yoon, J.A.; Han, Y.T. Total Synthesis of the Natural Pyridocoumarins Goniothaline A and B. Synthesis 2019, 51, 552–556. [Google Scholar] [CrossRef] [Green Version]
- Aslam, M.S.; Ahmad, M.A.; Mamat, A.S.; Ahmad, M.Z.; Salam, F. Goniothalamus: Phytochemical and Ethnobotanical Review. Rec. Adv. Biol. Med. 2016, 2, 34–47. [Google Scholar] [CrossRef]
- Levrier, C.; Balastrier, M.; Beattle, K.D.; Carroll, A.R.; Martin, F.; Choomuenwai, V.; Davis, R.A. Pyridocoumarin, aristolactam and aporphine alkaloids from the Australian rainforest plant Goniothalamus australis. Phytochemistry 2013, 86, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-L.; Dou, M.; Luo, Q.; Cheng, L.-Z.; Yan, Y.-M.; Li, R.-T.; Cheng, Y.-X. Racemic alkaloids from the fungus Ganoderma cochlear. Fitoterapia 2017, 116, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Markey, M.D.; Fu, Y.; Kelly, T.R. Synthesis of Santiagonamine. Org. Lett. 2007, 9, 3255–3257. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.M.; Zhang, Q.J.; Chen, R.Y.; Yu, D.Q. Four new alkaloids from Polyalthia nemoralis (Annonaceae). J. Asian Nat. Prod. Res. 2008, 10, 656–664. [Google Scholar] [CrossRef]
- Pang, M.; Lee, J.; Jeon, J.-H.; Song, I.-S.; Han, Y.T.; Choi, M.-K. Development of a Sensitive Analytical Method of Polynemoraline C Using LCMS/MS and Its Application to a Pharmacokinetic Study in Mice. Mass Spectrom. Lett. 2021, 12, 200–205. [Google Scholar] [CrossRef]
- Houghton, P.J.; Woldemariam, T.Z.; Khan, A.I.; Burke, A.; Mahmood, N. Antiviral activity of natural and semi-synthetic chromone alkaloids. Antivir. Res. 1994, 25, 235–244. [Google Scholar] [CrossRef]
- Houghton, P.J.; Hairon, Y. Further chromone alkaloids from Schumanniophyton magnificum. Planta Med. 1987, 53, 262–264. [Google Scholar] [CrossRef]
- Houghton, P.J. Revision of structures of some Schumanniophyton alkaloids. Planta Med. 1987, 53, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.J.; Hairong, Y. Novel Chromone Alkaloids from Schumanniophyton magnificum. Planta Med. 1985, 51, 23–27. [Google Scholar] [CrossRef]
- Darbavar, M.; Sundaramurthy, V. Synthesis of Coumarins with 3:4-Fused Ring Systems and their Physiological Activity. Synthesis 1982, 1982, 337–388. [Google Scholar] [CrossRef]
- Rawat, A.; Reddy, A.V.B. Recent advances on anticancer activity of coumarin derivatives. Eur. J. Med. Chem. Rep. 2022, 5, 100038. [Google Scholar] [CrossRef]
- Patra, P. A Concise Review on Pyridocoumarin/Azacoumarin Derivatives: Synthesis and Biological Activity. Chem. Sel. 2019, 4, 2024–2043. [Google Scholar] [CrossRef]
- Patra, P. 4-Chloro-3-formylcoumarin as a multifaceted building block for the development of various bio-active substituted and fused coumarin heterocycles: A brief review. New J. Chem. 2021, 45, 14269–14327. [Google Scholar] [CrossRef]
- Manske, R.H. The chemistry of quinolines. The Chemistry of Quinolines. Chem. Rev. 1942, 30, 113–144. [Google Scholar] [CrossRef]
- Manske, R.H.F.; Kulka, M. The Skraup Synthesis of Quinolines in Organic Reactions; John Wiley & Sons: New York, NY, USA, 1953; Volume 7, pp. 59–98. [Google Scholar]
- Dey, D.B.; Goswami, M.N. XXXIX. ψ-1:8-isoNaphthoxazone. J. Chem. Soc. 1919, 115, 531–541. [Google Scholar] [CrossRef]
- Liska, K.J.; Fentiman, A.F., Jr.; Foltz, R.L. Use of tris-(dipivalomethanato)europium as a shift reagent in the identification of 3-H-pyrano[3,2-f]quinoline-3-one. Tetrahedron Lett. 1970, 53, 4657–4660. [Google Scholar] [CrossRef]
- Denmark, S.E.; Venkatraman, S. On the Mechanism of the Skraup−Doebner−Von Miller Quinoline Synthesis. J. Org. Chem. 2006, 71, 1668–1676. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Liu, L.; Li, H.-J.; Wang, D.; Chen, Y.-J. Skraup−Doebner−Von Miller Quinoline Synthesis Revisited: Reversal of the Regiochemistry for γ-Aryl-β,γ-unsaturated α-Ketoesters. J. Org. Chem. 2006, 71, 6592–6595. [Google Scholar] [CrossRef] [PubMed]
- Heber, D.; Berghaus, T. Synthesis of 5H-[1]benzopyrano[4,3-b]pyridin-5-ones containing an azacannabinoidal structure. J. Heterocycl. Chem. 1994, 31, 1353–1359. [Google Scholar] [CrossRef]
- Yadav, A.; Biswas, S.; Mobin, S.M.; Samanta, S. Efficient Cu(OTf)2-catalyzed and microwave-assisted rapid synthesis of 3,4-fused chromenopyridinones under neat conditions. Tetrahedron Lett. 2017, 58, 3634–3639. [Google Scholar] [CrossRef]
- Osipov, D.V.; Artyomenko, A.A.; Osyanin, V.A.; Klimochkin, Y.N. The reaction of 4-aminocoumarin with β-carbonyl-substituted 4H-chromenes: Synthesis of 5H-chromeno [4,3-b] pyridin-5-one derivatives. Chem. Heterocycl. Comp. 2019, 55, 261–265. [Google Scholar] [CrossRef]
- Osipov, D.V.; Osyanin, V.A.; Klimochkin, Y.N. Synthesis of β-(o-hydroxybenzyl) pyridines by three-component condensation of ammonia, carbonyl-substituted 4H-chromenes, and CH acids. Chem. Heterocycl. Comp. 2018, 54, 1121–1126. [Google Scholar] [CrossRef]
- Povarov, L.S. α,β-Unsaturated ethers and their analogues in reactions of diene synthesis. Russ. Chem. Rev. 1967, 36, 656–670. [Google Scholar] [CrossRef]
- Kouznetsov, V.V. Recent synthetic developments in a powerful imino Diels–Alder reaction (Povarov reaction): Application to the synthesis of N-polyheterocycles and related alkaloids. Tetrahedron 2009, 65, 2721–2750. [Google Scholar] [CrossRef]
- Wang, X.-S.; Yin, M.-Y.; Wang, W.; Tu, S.-J. A Stereoselective Povarov Reaction Leading to exo-Tetrahydroindolo[3,2-c]quinoline Derivatives Catalyzed by Iodine. Eur. J. Org. Chem. 2012, 2012, 4811–4818. [Google Scholar] [CrossRef]
- Domingo, L.R.; Aurell, M.J.; Saez, J.A.; Mekelleche, S.M. Understanding the mechanism of the Povarov reaction. A DFT study. RSC Adv. 2014, 4, 25268–25278. [Google Scholar] [CrossRef] [Green Version]
- Clerigué, J.; Ramos, M.T.; Menéndez, J.C. Enantioselective catalytic Povarov reactions. Org. Biomol. Chem. 2022, 20, 1550–1581. [Google Scholar] [CrossRef]
- Kobayashi, S.; Ishitani, H.; Nagayama, S. Lanthanide Triflate Catalyzed Imino Diels-Alder Reactions; Convenient Syntheses of Pyridine and Quinoline Derivatives. Synthesis 1995, 1995, 1195–1202. [Google Scholar] [CrossRef]
- Ma, Y.; Qian, C.; Xie, M.; Sun, J. Lanthanide Chloride Catalyzed Imino Diels−Alder Reaction. One-Pot Synthesis of Pyrano[3,2-c]- and Furo[3,2-c]quinolines. J. Org. Chem. 1999, 64, 6462–6467. [Google Scholar] [CrossRef]
- Kudale, A.A.; Kendall, J.; Miller, D.O.; Collins, J.L.; Bodwell, G.J. Povarov Reactions Involving 3-Aminocoumarins: Synthesis of 1,2,3,4-Tetrahydropyrido[2,3-c]coumarins and Pyrido[2,3-c]coumarins. J. Org. Chem. 2008, 73, 8437–8447. [Google Scholar] [CrossRef]
- Kudale, A.A.; Miller, D.O.; Dawe, L.N.; Bodwell, G.J. Intramolecular Povarov reactions involving 3-aminocoumarins. Org. Biomol. Chem. 2011, 9, 7196–7206. [Google Scholar] [CrossRef]
- Islam, K.; Das, D.K.; Akram, E.; Khan, A.T. Exploration of C5–C6-Unsubstituted 1,4-Dihydropyridines for the Construction of exo-Hexahydro-1H-chromeno[3,4-h][1,6]naphthyridine-3-carboxylates Using a Stereoselective Povarov Reaction. Synthesis 2015, 47, 2745–2755. [Google Scholar] [CrossRef]
- Khan, A.T.; Das, D.K.; Islam, K.; Das, P. A simple and expedient synthesis of functionalized pyrido[2,3-c] coumarin derivatives using molecular iodine catalyzed three-component reaction. Tetrahedron Lett. 2012, 53, 6418–6422. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Ponra, S.; Ghosh, D.; Taher, A. Efficient one-pot synthesis of substituted 4, 7-phenanthroline, pyrano-[3, 2-f] quinoline and pyrano [3, 2-g] quinoline derivatives by aza-diels-alder reaction. Synth. Lett. 2011, 1, 104–110. [Google Scholar] [CrossRef]
- Symeonidis, T.S.; Litinas, K.E. Synthesis of methyl substituted [5,6]- and [7,8]-fused pyridocoumarins via the iodine-catalyzed reaction of aminocoumarins with n-butyl vinyl ether. Tetrahedron Lett. 2013, 54, 6517–6519. [Google Scholar] [CrossRef]
- Ganguli, N.C.; Chandra, S. One-pot access to pyridocoumarins via Povarov-hydrogen transfer cascade under auto-tandem catalysis of iodine in aqueous micelles. Tetrahedron Lett. 2014, 55, 1564–1568. [Google Scholar] [CrossRef]
- Symeonidis, T.S.; Hadjipavlou-Litina, D.J.; Litinas, K.E. Synthesis Through Three-Component Reactions Catalyzed by FeCl3 of Fused Pyridocoumarins as Inhibitors of Lipid Peroxidation. J. Heterocycl. Chem. 2014, 51, 642–647. [Google Scholar] [CrossRef]
- Das, D.K.; Sarkar, S.; Khan, A.T.; Saravanan, P.; Patra, S. Synthesis of fused tetrahydropyrido[2,3-c]coumarin derivatives as potential inhibitors for dopamine d3 receptors, catalyzed by hydrated ferric sulfate. RSC Adv. 2014, 4, 3581–3590. [Google Scholar] [CrossRef]
- Belal, M.; Das, D.K.; Khan, A.T. Synthesis of Pyrido[2,3-c]coumarin Derivatives by an Intramolecular Povarov Reaction. Synthesis 2015, 47, 1109–1116. [Google Scholar] [CrossRef]
- Xi, G.-L.; Liu, Z.-Q. Coumarin sharing the benzene ring with quinoline for quenching radicals and inhibiting DNA oxidation. Eur. J. Med. Chem. 2015, 95, 416–423. [Google Scholar] [CrossRef]
- Gurumurthy, C.; Fatima, N.; Reddy, G.N.; Kumar, C.G.; Sabitha, G.; Ramakrishna, K.V.S. A diastereoselective synthesis of tetrahydro- and dihydro-pyrido[2,3-c]coumarin derivatives via a one-pot three-component Povarov reaction catalyzed by bismuth(III) chloride. Bioorg. Med. Chem. Lett. 2016, 26, 5119–5125. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hu, L.; Peng, F. Efficient Synthesis of Functionalized Pyrido[2,3-c]coumarin Derivatives by a One-Pot Three-Component Reaction. Synth. Lett. 2016, 27, 1888–1892. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.-C.; Yan, S.-J. The Friedlander Synthesis of Quinolines in Organic Reactions; John Wiley & Sons: New York, NY, USA, 1982; Volume 28, pp. 37–201. [Google Scholar]
- Marco-Contelles, J.; Pérez-Mayoral, E.; Samadi, A.; do Carmo Carreiras, M.; Soriano, E. Recent Advances in the Friedländer Reaction. Chem. Rev. 2009, 109, 2652–2671. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, N.; Nazari, N.; Gholamzadeh, P. The Friedlander reaction: A powerful strategy for the synthesis of heterocycles. Adv. Heterocycl. Chem. 2020, 132, 85–134. [Google Scholar] [CrossRef]
- Siddiqui, Z.N.; Khan, K. Friedlander synthesis of novel benzopyranopyridines in the presence of chitosan as heterogeneous, efficient and biodegradable catalyst under solvent-free conditions. New J. Chem. 2013, 39, 1595–1602. [Google Scholar] [CrossRef]
- Suzuki, M.; Yu, D.; Morris-Natschke, S.L.; Smith, P.C.; Lee, K.-H. Anti-AIDS agents 66: Syntheses and anti-HIV activity of phenolic and aza 3′,4′-di-O-(−)-camphanoyl-(+)-cis-khellactone (DCK) derivatives. Bioorg. Med. Chem. 2007, 15, 6852–6858. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Ansary, I.; Samanta, S.; Roy, B.; Ansary, I.; Samanta, S.; Roy, B. Regioselective synthesis of pyridoquinolones and pyridocoumarins via molecular iodine-mediated 6-endo-dig electrophilic cylization. Tetrahedron Lett. 2011, 52, 411–414. [Google Scholar] [CrossRef]
- Symeonidis, T.S.; Kallitsakis, M.G.; Litinas, K.E. Synthesis of [5,6]-fused pyridocoumarins through aza-Claisen rearrangement of 6-propargylaminocoumarins. Tetrahedron Lett. 2011, 52, 5452–5455. [Google Scholar] [CrossRef]
- Symeonidis, T.S.; Lykakis, I.N.; Litinas, K.E. Synthesis of quinolines and fused pyridocoumarins from N-propargylanilines or propargylaminocoumarins by catalysis with gold nanoparticles supported on TiO2. Tetrahedron 2013, 69, 4612–4616. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Ponra, S. Regioselective Synthesis of Dihydropyridocoumarin and Phenanthrolinone Derivatives via Iron(III) Chloride Mediated Intramolecular Cyclization. Synthesis 2014, 46, 1413–1420. [Google Scholar] [CrossRef]
- Yoon, Y.A.; Ha, Y.T. Efficient Synthesis of Pyrido[3,2-c]coumarins via Silver Nitrate Catalyzed Cycloisomerization and Application to the First Synthesis of Polyneomarline C. Synthesis 2019, 51, 4611–4618. [Google Scholar] [CrossRef]
- Vlachou, E.-E.N.; Fotopoulos, I.; Gabriel, C.; Pontiki, E.; Hadjipavlou-Litina, D.J.; Litinas, K.E. Synthesis and biological evaluation of fused dipyranoquinolinones as inhibitors of acetylcholinesterase with antioxidant properties. Eur. J. Med. Chem. Rep. 2022, 5, 100063. [Google Scholar] [CrossRef]
- Zhu, J.; Bienaymé, H. (Eds.) Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Dömling, A.; Wang, W.; Wang, K. Chemistry and Biology of Multicomponent Reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef] [Green Version]
- da Silveira Pinto, L.S.; Couri, M.R.C.; de Souza, M.V.N. Multicomponent Reactions in the Synthesis of Complex Fused Coumarin Derivatives. Curr. Org. Synth. 2018, 15, 21–37. [Google Scholar] [CrossRef]
- Bhaskaruni, S.V.H.S.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A review on multi-component green synthesis of N-containing heterocycles using mixed oxides as heterogeneous catalysts. Arab. J. Chem. 2020, 13, 1142–1178. [Google Scholar] [CrossRef]
- John, S.E.; Gulati, S.; Shankaraiah, N. Recent advances in multi-component reactions and their mechanistic insights: A triennium review. Org. Chem. Front. 2021, 8, 4237–4287. [Google Scholar] [CrossRef]
- Khan, A.T.; Das, D.K. Michael Initiated Ring Closure (MIRC) reaction on in situ generated benzylidenecyclohexane-1,3-diones for the construction of chromeno[3,4-b]quinoline derivatives. Tetrahedron Lett. 2012, 53, 2345–2351. [Google Scholar] [CrossRef]
- Paul, S.; Das, A.R. An efficient green protocol for the synthesis of coumarin fused highly decorated indenodihydropyridyl and dihydropyridyl derivatives. Tetrahedron Lett. 2012, 53, 2206–2210. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Paul, S.; Das, A.R.E. Facile synthesis of pyridopyrimidine and coumarin fused pyridine libraries over a Lewis base-surfactant-combined catalyst TEOA in aqueous medium. RSC. Adv. 2013, 3, 3203–3208. [Google Scholar] [CrossRef]
- Patra, P.; Kar, G.K.; Khatua, B. Thermolysis of N-Aryl Enaminoimine Hydrochloride Derivatives: A Short and General Method for the Synthesis of Pyranoquinolin-3-one and Pyranoacridin-3-one Derivatives. J. Heterocycl. Chem. 2014, 51, 1306–1310. [Google Scholar] [CrossRef]
- Patra, P. Thermolysis of Chlorovinyl Imines as an Alternate Route for the Synthesis of Pyranoquinolin-3-one and Pyranoacridin-3-one Derivatives. J. Heterocycl. Chem. 2017, 54, 3656–3662. [Google Scholar] [CrossRef]
- Kausar, N.; Das, A.R. CuI–Zn(OAc)2 catalyzed C(sp2)–H activation for the synthesis of pyridocoumarins through an uncommon CuI–CuIII switching mechanism: A fast, solvent-free, combo-catalytic, ball milling approach. Tetrahedron Lett. 2017, 58, 2602–2607. [Google Scholar] [CrossRef]
- Najafizadeh, F.; Rad-Moghadam, K.; Kalurazi, S.Y. A derivatization-directed three-component synthesis of fluorescent spiro [dihydropyridine-4,3ʹ-indoline]s. J. Chem. Res. 2020, 44, 527–531. [Google Scholar] [CrossRef]
- Oshiro, P.B.; Bregadiolli, B.A.; da Silva-Filho, L.C. A facile one-step synthesis of chromeno[4,3-b]pyridine derivatives promoted by niobium pentachloride. J. Heterocycl. Chem. 2020, 57, 2795–2800. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Chattopadhyay, B.; Nath, S. New Heck coupling strategies for the arylation of secondary and tertiary amides via palladium-catalyzed intramolecular cyclization. Tetrahedron Lett. 2008, 49, 1609–1612. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Nandi, R.K.; Ponra, S. Indium (III) Chloride Catalyzed Convergent, Regioselective Synthesis of Annulated Quinoline and Pyridine Derivatives. Synth. Lett. 2012, 23, 113–119. [Google Scholar] [CrossRef]
- Nath, S. Synthesis of pyridocoumarin derivative by arylation of tertiary and secondary amide via Palladium catalyzed intramolecular cyclization. J. Appl. Chem. 2017, 10, 80–85. [Google Scholar] [CrossRef]
- Weng, Y.; Zhou, H.; Sun, C.; Xie, Y.; Su, W. Copper-Catalyzed Cyclization for Access to 6H-Chromeno[4,3-b]quinolin-6-ones Employing DMF as the Carbon Source. J. Org. Chem. 2017, 82, 9047–9053. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Chen, H.; Li, N.; Yang, L.; Ackermann, L. Electrooxidative Metal-Free Cyclization of 4-Arylaminocoumarins with DMF as C1-Source. Adv. Synth. Catal. 2021, 363, 2773–2777. [Google Scholar] [CrossRef]
- Khan, E.; Biswas, S.; Mishra, S.K.; Mishra, R.; Samanta, S.; Mishra, A.; Tawani, A.; Kumar, A. Rationally designed small molecules targeting toxic CAG repeat RNA that causes Huntington’s disease (HD) and spinocerebellar ataxia (SCAs). Biochimie 2019, 163, 21–32. [Google Scholar] [CrossRef]
- Kidwai, M.; Rastogi, S.; Mohan, R. A Novel Route to New Bis(benzopyrano) Fused Dihydropyridines Using Dry Media. Bull. Korean J. Chem. 2004, 25, 119–121. [Google Scholar] [CrossRef] [Green Version]
- Tu, S.; Wu, S.; Yan, S.; Hao, W.; Zhang, X.; Cao, X.; Han, Z.; Jiang, B.; Shi, F.; Xia, M.; et al. Design and Microwave-Assisted Synthesis of Naphtho[2,3-f]quinoline Derivatives and Their Luminescent Properties. J. Comb. Chem. 2009, 11, 239–242. [Google Scholar] [CrossRef]
- Tu, S.; Zhang, Y.; Zhang, J.; Jiang, B.; Jia, R.; Zhang, J.; Ji, S. A Simple Procedure for the Synthesis of 4-Aza-podophyllotoxin Derivatives in Water under Microwave Irradiation Conditions. Synlett 2006, 17, 2785–2790. [Google Scholar] [CrossRef]
- Miri, R.; Motamedi, R.; Rezaei, M.R.; Firuzi, O.; Javidnia, A.; Shafiee, A. Design, Synthesis and Evaluation of Cytotoxicity of Novel Chromeno[4,3-b]quinoline Derivatives. Arch. Pharm. Chem. Life Sci. 2011, 2, 111–118. [Google Scholar] [CrossRef]
- Chen, Z.; Bi, J.; Su, W. Synthesis and Antitumor Activity of Novel Coumarin Derivatives via a Three-component Reaction in Water. Chin. J. Chem. 2013, 31, 507–514. [Google Scholar] [CrossRef]
- Khan, M.N.; Pal, S.; Karamthulla, S.; Choudhury, L.H. Multicomponent reactions for facile access to coumarin-fused dihydroquinolines and quinolines: Synthesis and photophysical studies. New J. Chem. 2014, 38, 4722–4729. [Google Scholar] [CrossRef] [Green Version]
- Dam, B.; Nandi, S.; Pal, A.K. An efficient ‘on-water’ synthesis of 1,4-dihydropyridines using Fe3O4@SiO2 nanoparticles as a reusable catalyst. Tetrahedron Lett. 2014, 55, 5236–5240. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Palnati, G.R.; Singh, L.R.; Upadhyay, A.; Avula, S.R.; Kumara, A.; Kant, R. Molecular iodine catalysed one-pot synthesis of chromeno[4,3-b]quinolin-6-ones under microwave irradiation. Green Chem. 2015, 17, 3766–3770. [Google Scholar] [CrossRef]
- Liu, M.; Yin, G.; Zhu, C.; Yao, C. Selective Synthesis of New Tetracyclic Coumarin-fused Pyrazolo[3,4-b]pyridines and Pyrazolo[3,4-b]pyridin-6(7H)-ones. J. Heterocycl. Chem. 2016, 53, 1617–1625. [Google Scholar] [CrossRef]
- Kumari, S.; Rajeswari, M.; Khurana, J.M. A Green Approach for the Synthesis of Novel 7, 11-Dihydro-6H-chromeno [3, 4-e] isoxazolo [5,4-b] pyridin-6-one Derivatives Using Acidic Ionic Liquid [C4mim][HSO4]. Aust. J. Chem. 2016, 69, 1049–1053. [Google Scholar] [CrossRef]
- Rahimzadeh, G.; Bahadorikhalili, S.; Kianmehr, E.; Mahdavi, M. Ionic liquid-functionalized magnetic nanostructures as an efficient catalyst for the synthesis of 6H-chromeno[4,3-b]quinolin-6-ones. Mol. Divers. 2017, 21, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Firoozpour, L.; Nikookar, H.; Moghimi, S.; Mahdavi, M.; Asadipour, A.; Ranjbar, P.R.; Foroumadi, A. An efficient approach to the synthesis of coumarin-fused dihydropyridinones. J. Heterocycl. Commun. 2017, 23, 305–308. [Google Scholar] [CrossRef]
- Sayahi, M.H.; Saghanezhad, S.J.; Mahdavi, M. SBA-15-SO3H-assisted preparation of 4-aza-phenanthrene-3, 10-dione derivatives via a one-pot, four-component reaction. Res. Chem. Interm. 2018, 44, 739–747. [Google Scholar] [CrossRef]
- Ataee-Kachouei, T.; Nasr-Esfahani, M.; Mohammadpoor-Baltork, I.; Mirkhani, V.; Moghadam, M.; Tangestaninejad, S.; Kia, R. Facile and Green One-Pot Synthesis of Fluorophore Chromeno[4,3-b]quinolin-6-one Derivatives Catalyzed by Halloysite Nanoclay under Solvent-free Conditions. ChemistrySelect 2019, 4, 2301–2306. [Google Scholar] [CrossRef]
- Zeynizadeh, B.; Rahmani, S. Sulfonyl-bridged (copper-immobilized nickel ferrite) with activated montmorillonite, [(NiFe2O4@Cu)SO2(MMT)]: A new class of magnetically separable clay nanocomposite systems towards Hantzsch synthesis of coumarin-based 1,4-dihydropyridines. RSC Adv. 2019, 9, 8002–8015. [Google Scholar] [CrossRef] [Green Version]
- Zeynizadeh, B.; Rahmani, S.; Eghbali, E. Anchored sulfonic acid on silica-layered NiFe2O4: A magnetically reusable nanocatalyst for Hantzsch synthesis of 1,4-dihydropyridines. Polyhedron 2019, 168, 57–66. [Google Scholar] [CrossRef]
- Singha, R.; Islam, A.; Ghosh, P. One-pot three-component tandem annulation of 4-hydroxycoumarine with aldehyde and aromatic amines using graphene oxide as an efficient catalyst. Sci. Rep. 2021, 11, 19891. [Google Scholar] [CrossRef]
- Jamshaid, S.; Mohandoss, S.; Lee, Y.R. Indium(iii)-catalyzed solvent-free multicomponent [2 + 2 + 1 + 1]-annulation to polycyclic functionalized fused pyridines as potential optical chemosensors. Green Chem. 2021, 23, 5113–5119. [Google Scholar] [CrossRef]
- Pave, G.; Ghalard, P.; Viaud-Massuard, M.-C.; Troin, Y.; Guillaumet, G. New efficient synthesis of pyrido [2, 3-c] and pyrido [3, 2-c] coumarin derivatives. Synlett 2003, 7, 987–990. [Google Scholar] [CrossRef]
- Zecher, W.; Krohnke, F. Eine neue Synthese substituierter Pyridine, I. Grundzüge der Synthese. Chem. Ber. 1961, 94, 690–697. [Google Scholar] [CrossRef]
- Krohnke, F.; Zecher, W.; Kurtze, J.; Drechsler, D.; Pfleghar, K.; Schnalke, K.E.; Weis, W. Syntheses Using the Michael Adddition of Pyridinium Salts. Angew. Chem. Internat. Edit. 1962, 1, 626–632. [Google Scholar] [CrossRef]
- Krohnke, F. The Specific Synthesis of Pyridines and Oligopyridines. Synthesis 1976, 1976, 1–24. [Google Scholar] [CrossRef]
- Pandya, S.; Pandya, U.R.; Hirani, B.R.; Brahmbhatt, D.I. One pot synthesis of diarylpyrido[3,2-c]coumarins. J. Heterocycl. Chem. 2006, 43, 795–798. [Google Scholar] [CrossRef]
- Brahmbhatt, D.I.; Patel, N.H.; Patel, A.K.; Patel, M.A.; Patel, V.G. Synthesis and antimicrobial activity of some 7-aryl-5,6-dihydro-14-aza[1]benzopyrano[3,4-b]phenanthren-8H-ones. J. Heterocycl. Chem. 2011, 48, 840–844. [Google Scholar] [CrossRef]
- Sharma, D.; Sharma, N.; Patel, N.H.; Brahmbhatt, D.I.; Gupta, V.K. Crystal Structure of 7-Phenyl-5, 6-Dihydro-14-Aza-[1]benzopyrano [3, 4-b] phenanthren-8H-One. Crystallogr. Rep. 2021, 66, 1223–1226. [Google Scholar] [CrossRef]
- Rao, Y.; Liu, M.; Wu, L.; Yin, G. Catalyst-free one-pot domino reactions for selective synthesis of functionalized 2,8-oxazabicyclo[3.3.1]-nonanes and 5H-indeno[1,2-b]pyridin-5-ones. RSC Adv. 2014, 4, 64551–64558. [Google Scholar] [CrossRef]
- Giri, R.R.; Brahmbhadtt, D.I. A Convenient Synthesis of Bipyrido-Fused Coumarins and Their Biological Evaluation. J. Heterocycl. Chem. 2019, 56, 2630–2636. [Google Scholar] [CrossRef]
- Al-Omran, F.; Elassar, A.-Z.A.; ElKhair, A.A. Synthesis and biological effects of new derivatives of azines incorporating coumarin. J. Heterocycl. Chem. 2003, 40, 249–254. [Google Scholar] [CrossRef]
- Ismail, M.M.F.; Noaman, E. Novel Pirfenidone Analogs as Antifibrotic Agents: Synthesis and Antifibrotic Evaluation of 2-Pyridones and Fused Pyridones. Med. Chem. Res. 2005, 14, 382–403. [Google Scholar] [CrossRef]
- Khan, I.A.; Kulkarni, M.V.; Gopal, M.; Shahabuddin, M.S.; Sun, C.-M. Synthesis and biological evaluation of novel angularly fused polycyclic coumarins. Bioorg. Med. Chem. Lett. 2005, 15, 3584–3587. [Google Scholar] [CrossRef] [PubMed]
- Glasnov, T.N.; Ivanov, I.C. A Convenient Approach to the Synthesis of Dialkyl 5-Oxo-1,2-dihydro-5H-chromeno [4,3-b]pyridine-2,3-dicarboxylates. Synth. Commun. 2008, 38, 1579–1588. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Debnath, P.; Chottopadhyay, S.K. Aryl Radical Cyclization: Regioselective Synthesis of 6a,7,8,12b-Tetrahydro-6H-chromeno[3,4-c]quinolin-6-one. Synth. Commun. 2008, 38, 1768–1777. [Google Scholar] [CrossRef]
- Jadhav, V.B.; Nayak, S.K.; Guru Row, T.N.; Kulkarni, M.V. Synthesis, structure and DNA cleavage studies of coumarin analogues of tetrahydroisoquinoline and protoberberine alkaloids. Eur. J. Med. Chem. 2010, 45, 3575–3580. [Google Scholar] [CrossRef] [PubMed]
- El-Said, M.S.; El-Gazzar, M.G.; Al-Dosari, M.S.; Ghorab, M.M. Synthesis, anticancer activity and radiosensitizing evaluation of some new 2-pyridone derivatives. Arzneimittelforschung 2012, 62, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Hu, X.; Song, S.; Cai, Q.; Wang, Y.; Shi, D. Microwave-assisted synthesis of novel hetero[5]helicene-like molecules and coumarin derivatives. Org. Biomol. Chem. 2017, 15, 7909–7916. [Google Scholar] [CrossRef] [Green Version]
- Borah, P. Synthesis of some novel annulated coumarins by exploring intramolecular Hetero Diels-Alder reaction strategy. J. Appl. Fundam. Sci. 2018, 4, 81–83. [Google Scholar]
- Li, J.; Yang, Q.; Chen, G. Synthesis method of (3,4-dihydro-2H-pyrano[2,3-b]pyridin-6-yl)methanol. Patent No. CN 110283180 (A), 27 September 2019. [Google Scholar]
- Pandya, M.K.; Parekh, T.H.; Chhasatia, M.R.; Vala, N.D. Synthesis and characterization of some novel coumarin based various 2-aryl-pyrido [3,2-c] coumarins. J. Drug Deliv. Therap. 2020, 10, 158–163. [Google Scholar] [CrossRef]
- Heber, D.; Ivanov, I.C.; Karagiosov, S.K. The vilsmeier reaction in the synthesis of 3-substituted [1]benzopyrano[4,3-b]pyridin-5-ones. An unusual pyridine ring closure. J. Heterocycl. Chem. 1995, 32, 505–509. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X. Facile synthesis of chromeno[4,3-b]quinolin-6-ones from unexpected reactions of aryl isocyanides with 4-chloro-2-oxo-2H-chromene-3-carbaldehyde. Org. Biomol. Chem. 2006, 4, 1348–1351. [Google Scholar] [CrossRef]
- Prasad, J.V.; Reddy, J.S.; Kumar, N.R.; Solomon, K.A.; Gopikrishna, G. An efficient ultrasound promoted catalyst-free protocol for the synthesis of chromeno[4,3-b]quinolin-6-ones. J. Chem. Sci. 2011, 123, 673–679. [Google Scholar] [CrossRef]
- Borah, P.; Naidu, P.S.; Bhuyan, P.J. Synthesis of some tetrazole fused pyrido[2,3-c]coumarin derivatives from a one-pot three-component reaction via intramolecular 1,3-dipolar cycloaddition reaction of azide to nitriles. Tetrahedron Lett. 2012, 53, 5034–5037. [Google Scholar] [CrossRef]
- Halawa, A.H.; El-Gilil, S.M.A.; Bedair, A.H.; Eliwa, E.M.; Frese, M.; Sewald, N.; Shaaban, M.; El-Agrody, A.M. Synthesis of diverse amide linked bis-indoles and indole derivatives bearing coumarin-based moiety: Cytotoxicity and molecular docking investigations. Med. Chem. Res. 2018, 27, 796–806. [Google Scholar] [CrossRef]
- Kolita, S.; Bhuyan, P.J. An Efficient Synthesis of Pyrido[3,2-c]coumarins under Microwave Irradiation in Solvent-Free Conditions. ChemistrySelect 2018, 3, 1411–1414. [Google Scholar] [CrossRef]
- Iarohsenko, V.O.; Erben, F.; Mrktchyan, S.; Hakobyan, A.; Vilches-Herrera, M.; Dudkin, S.; Bunescu, A.; Villiger, A.; Sosnovskikh, V.Y.; Lange, P. 4-Chloro-3-(trifluoroacetyl)- and 4-chloro-3-(methoxalyl)coumarins as novel and efficient building blocks for the regioselective synthesis of 3,4-fused coumarins. Tetrahedron 2011, 67, 7946–7955. [Google Scholar] [CrossRef]
- Becalli, E.M.; Contini, A.; Trimarco, P. New synthetic approach to [1]benzopyrano[4,3-b]pyridin-5-one derivatives. Tetrahedron Lett. 2004, 45, 3447–3449. [Google Scholar] [CrossRef]
- Khan, H.J.; Petrow, V.; Rewald, E.L.; Sturgeon, B. 452. Some derivatives of 1:3-dimethyl-2-azafluorenone. J. Chem. Soc. 1949, 452, 2128–2134. [Google Scholar] [CrossRef]
- Courts, A.; Petrow, V. 69. New syntheses of heterocyclic compounds. Part XV. 9: 10-Dihydro-10-keto-1:3-dimethyl-2-aza-9-oxaphenanthrenes. J. Chem. Soc. 1952, 200, 334–337. [Google Scholar] [CrossRef]
- Pars, H.G.; Grancelli, F.E.; Keller, J.K.; Razdan, R.K. Physiologically Active Nitrogen Analogs of Tetrahydrocannabinols. Tetrahydrobenzopyrano[3,4-d]pyridines. J. Am. Chem. Soc. 1966, 88, 3664–3665. [Google Scholar] [CrossRef]
- Mandal, T.K.; Soldatenkov, A.T.; Ageev, E.A.; Stashenko, E.V.; Denisov, E.N.; Andreeva, E.I.; Prostakov, N.S. Synthesis and fungicidal activity of substituted tetrahydro-[3,4-c]- and benzo[h]tetrahydropyrido[3,4-c]coumarins. Pharm. Chem. J. 1990, 24, 145–149. [Google Scholar] [CrossRef]
- Abramovitch, R.A.; Inbasekaran, M.N.; Kato, S.; Radzikowska, T.A.; Tomasik, P. Base-catalyzed rearrangement of N-(aryloxy)pyridinium salts. Effect of a 3-substituent in the pyridine ring upon orientation. Synthesis of novel tricyclic rings. J. Org. Chem. 1983, 48, 690–695. [Google Scholar] [CrossRef]
- Galariniotou, E.; Fragos, V.; Makri, A.; Litinas, K.E.; Nicolaides, D.N. Synthesis of novel pyridocoumarins and benzo-fused 6-azacoumarins. Tetrahedron 2007, 63, 8298–8304. [Google Scholar] [CrossRef]
- Yavari, I.; Adib, M.; Hojabri, L. Vinyltriphenylphosphonium salt mediated synthesis of 1,4-benzoxazine and coumarin derivatives. Tetrahedron 2002, 58, 6895–6899. [Google Scholar] [CrossRef]
- El-Saghier, A.M.; Mo, E.-J.I.; Al-Afaleq, E.-J.I. Synthetic Reactions of Coumarin-3-(4- Aminosulfonyl)-Carbanilide Derivatives With Reactive Methylene Compounds. Phosphorus Sulfur Silicon 1998, 139, 67–75. [Google Scholar] [CrossRef]
- Sviripa, V.M.; Fiandalo, M.V.; Begley, K.L.; Wyrebek, P.; Kril, L.M.; Balia, A.G.; Parkin, S.R.; Subramanian, V.; Chen, X.; Williams, A.H.; et al. Pictet–Spengler condensations using 4-(2-aminoethyl)coumarins. New J. Chem. 2020, 44, 13415–13429. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, A.; Midorikawa, H. Condensation of active methylene compounds with hydroxybenzaldehydes by ammonium acetate. J. Org. Chem. 1969, 34, 3612–3615. [Google Scholar] [CrossRef]
- Sakurai, A.; Midorikawa, H.; Hashimoto, Y. The cyclization of ethyl cyanoacetate and salicylaldehyde or 3-methoxysalicylaldehyde with ketones by means of ammonium acetate. Bull. Chem. Soc. Jpn. 1970, 43, 2925–2933. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, A.; Midorikawa, H.; Hashimoto, Y. Substituted Benzopyranopyridine and Pyrimidine Ring Syntheses by the Ternary Condensation of Ethyl Cyanoacetate, Salicylaldehyde, and Certain Aldehydes in the presence of ammonium acetate. Bull. Chem. Soc. Jpn. 1971, 44, 1677–1682. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, A.; Motomura, Y.; Midorikawa, H. Substituted benzopyranopyridopyrimidine ring syntheses by the ternary condensation of malononitrile, salicylaldehyde, and aromatic ketones in the presence of ammonium acetate. J. Org. Chem. 1972, 37, 1523–1526. [Google Scholar] [CrossRef]
- O’Callaghan, M. Synthesis of Dialkyl 2-(2-Hydroxyphenyl)-4,6-dimethyl-1,2-dihydropyridine-3,5-dicarboxylates and Alkyl 2,4-Dimethyl-5-oxo-5H-[1]benzopyrano[4,3-b]-pyridine-3-carboxylates. Synthesis 1987, 1987, 499–503. [Google Scholar] [CrossRef]
- Navarrete-Encina, P.; Salazar, R.; Vega-Retter, C.; Perez, K.; Squella, J.A.; Nunez-Vergara, L.J. On the one pot syntheses of chromeno [4, 3-b] pyridine-3-carboxylate and chromeno [3, 4-c] pyridine-3-carboxylate and dihydropyridines. J. Braz. Chem. Soc. 2010, 21, 413–418. [Google Scholar] [CrossRef]
- Frolova, L.V.; Malik, I.; Uglinskii, P.Y.; Rogelj, S.; Kornienko, A.; Magedov, I.V. Multicomponent synthesis of 2,3-dihydrochromeno[4,3-d]pyrazolo[3,4-b]pyridine-1,6-diones: A novel heterocyclic scaffold with antibacterial activity. Tetrahedron Lett. 2011, 52, 6643–6645. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Riyadh, S.M. Multicomponent Synthesis of Novel Penta-Heterocyclic Ring Systems Incorporating a Benzopyranopyridine Scaffold. Synthesis 2014, 46, 258–262. [Google Scholar] [CrossRef]
- Palacios, F.; Alonso, C.; Amezua, P.; Rubiales, G. Synthesis of Aza Polycyclic Compounds Derived from Pyrrolidine, Indolizidine, and Indole via Intramolecular Diels−Alder Cycloadditions of Neutral 2-Azadienes. J. Org. Chem. 2002, 67, 1941–1946. [Google Scholar] [CrossRef] [PubMed]
- Keskin, S.; Balci, M. Intramolecular Heterocyclization of O-Propargylated Aromatic Hydroxyaldehydes as an Expedient Route to Substituted Chromenopyridines under Metal-Free Conditions. Org. Lett. 2015, 17, 964–967. [Google Scholar] [CrossRef] [PubMed]













































































































Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Douka, M.D.; Litinas, K.E. An Overview on the Synthesis of Fused Pyridocoumarins with Biological Interest. Molecules 2022, 27, 7256. https://doi.org/10.3390/molecules27217256
Douka MD, Litinas KE. An Overview on the Synthesis of Fused Pyridocoumarins with Biological Interest. Molecules. 2022; 27(21):7256. https://doi.org/10.3390/molecules27217256
Chicago/Turabian StyleDouka, Matina D., and Konstantinos E. Litinas. 2022. "An Overview on the Synthesis of Fused Pyridocoumarins with Biological Interest" Molecules 27, no. 21: 7256. https://doi.org/10.3390/molecules27217256
APA StyleDouka, M. D., & Litinas, K. E. (2022). An Overview on the Synthesis of Fused Pyridocoumarins with Biological Interest. Molecules, 27(21), 7256. https://doi.org/10.3390/molecules27217256