Cytoprotective Polyketides from Sponge-Derived Fungus Lopadostoma pouzarii
Abstract
:1. Introduction
2. Results
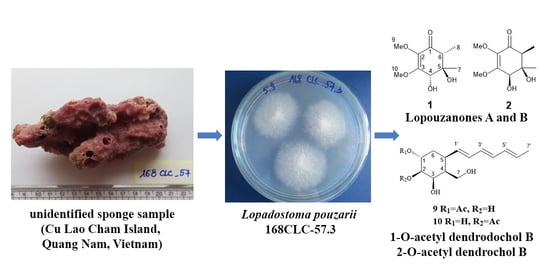
2.1. Isolation and Identification of Compounds 1–10
2.2. Biological Activity of Isolated Compounds
3. Discussion
4. Materials and Methods
4.1. General
4.2. Fungal Material and Fermentation
4.3. Extraction and Isolation
4.4. Isolated Compounds
4.5. DPPH Scavenging Assay
4.6. Bioassays
4.6.1. Cell Culture
4.6.2. Cell Viability Assay
4.6.3. Cardioprotective Activity of Compounds in CoCl2-Mimic Hypoxia
4.6.4. Cardioprotective Activity of Compounds against Rotenone-Induced Toxicity
4.7. Statistical Data Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2022, 39, 1122–1171. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.M.; Tang, X.L.; Sun, Y.T.; Song, D.Y.; Cheng, Y.J.; Liu, H.; Li, P.L.; Li, G.Q. Biological and chemical diversity of marine sponge-derived microorganisms over the last two decades from 1998 to 2017. Molecules 2020, 25, 853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2020, 37, 175–223. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Schmidt, E.W. Parallel lives of symbionts and hosts: Chemical mutualism in marine animals. Nat. Prod. Rep. 2018, 35, 357–378. [Google Scholar] [CrossRef]
- Bian, C.; Wang, J.; Zhou, X.; Wu, W.; Guo, R. Recent Advances on Marine Alkaloids from Sponges. Chem. Biodivers. 2020, 17, e2000186. [Google Scholar] [CrossRef]
- Zhang, B.; Higuchi, R.; Miyamoto, T.; Soest, R.W.M.V. Neuritogenic Activity-Guided Isolation of a Free Base Form Manzamine A from a Marine Sponge, Acanthostrongylophora aff. ingens (Thiele, 1899). Chem. Pharm. Bull. 2008, 56, 866–869. [Google Scholar] [CrossRef] [Green Version]
- Waters, A.L.; Peraud, O.; Kasanah, N.; Sims, J.W.; Kothalawala, N.; Anderson, M.A.; Abbas, S.H.; Rao, K.V.; Jupally, V.R.; Kelly, M.; et al. An analysis of the sponge Acanthostrongylophora igens microbiome yields an actinomycete that produces the natural product manzamine A. Front. Mar. Sci. 2014, 1, 54. [Google Scholar] [CrossRef] [Green Version]
- McKay, M.J.; Carroll, A.R.; Quinn, R.J.; Hooper, J.N.A. 1, 2-Bis (1 H-indol-3-yl) ethane-1, 2-dione, an Indole Alkaloid from the Marine Sponge Smenospongia sp. J. Nat. Prod. 2002, 65, 595–597. [Google Scholar] [CrossRef]
- Asiri, I.A.M.; Badr, J.M.; Youssef, D.T.A. Penicillivinacine, antimigratory diketopiperazine alkaloid from the marine-derived fungus Penicillium vinaceum. Phytochem. Lett. 2015, 13, 53–58. [Google Scholar] [CrossRef]
- Granmo, A.; Petrini, L.E. A new species of Lopadostoma, and the anamorph of Biscogniauxia cinereolilacina. Mycol. Helv. 1996, 8, 43–50. [Google Scholar]
- Jaklitsch, W.M.; Fournier, J.; Rogers, J.D.; Voglmayr, H. Phylogenetic and taxonomic revision of Lopadostoma. Pers.-Mol. Phylogeny Evol. Fungi 2014, 32, 52–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helaly, S.E.; Thongbai, B.; Stadler, M. Diversity of biologically active secondary metabolites from endophytic and saprotrophic fungi of the ascomycete order Xylariales. Nat. Prod. Rep. 2018, 35, 992–1014. [Google Scholar] [CrossRef] [PubMed]
- Quang, D.N.; Hashimoto, T.; Fournier, J.; Stadler, M.; Radulović, N.; Asakawa, Y. Sassafrins A–D, new antimicrobial azaphilones from the fungus Creosphaeria sassafras. Tetrahedron 2005, 61, 1743–1748. [Google Scholar] [CrossRef]
- Smetanina, O.F.; Yurchenko, A.N.; Ivanets, E.V.; Gerasimenko, A.V.; Trinh, P.T.H.; Ly, B.M.; Nhut, N.D.; Van, T.T.T.; Yurchenko, E.A.; Afiyatullov, S.S. Aromatic Metabolites of Marine Fungus Penicillium sp. KMM 4672 Associated with a Brown Alga Padina sp. Chem. Nat. Compd. 2017, 53, 600–602. [Google Scholar] [CrossRef]
- Yurchenko, A.N.; Trinh, P.T.H.; Girich, E.V.; Smetanina, O.F.; Rasin, A.B.; Popov, R.S.; Dyshlovoy, S.A.; von Amsberg, G.; Menchinskaya, E.S.; Van, T.T.T.; et al. Biologically Active Metabolites from the Marine Sediment-Derived Fungus Aspergillus flocculosus. Mar. Drugs 2019, 17, 579. [Google Scholar] [CrossRef] [Green Version]
- Kusumi, T.; Ooi, T.; Ohkubo, Y.; Yabuuchi, T. The modified Mosher’s method and the sulfoximine method. Bull. Chem. Soc. Jpn. 2006, 79, 965–980. [Google Scholar] [CrossRef]
- Xu, D.X.; Sun, P.; Kurtán, T.; Mándi, A.; Tang, H.; Liu, B.; Gerwick, W.H.; Wang, Z.W.; Zhang, W. Polyhydroxy cyclohexanols from a Dendrodochium sp. fungus associated with the sea cucumber holothuria Nobilis selenka. J. Nat. Prod. 2014, 77, 1179–1184. [Google Scholar] [CrossRef]
- Grove, J.F. The structure of gliorosein. J. Chem. Soc. C Org. 1966, 985. [Google Scholar] [CrossRef]
- Shushni, M.A.M.; Singh, R.; Mentel, R.; Lindequist, U. Balticolid: A new 12-membered macrolide with antiviral activity from an Ascomycetous fungus of marine origin. Mar. Drugs 2011, 9, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Xu, D.X.; Mándi, A.; Kurtán, T.; Li, T.J.; Schulz, B.; Zhang, W. Structure, absolute configuration, and conformational study of 12-membered macrolides from the fungus dendrodochium sp. associated with the sea cucumber holothuria nobilis selenka. J. Org. Chem. 2013, 78, 7030–7047. [Google Scholar] [CrossRef] [PubMed]
- Kokubun, T.; Veitch, N.C.; Bridge, P.D.; Simmonds, M.S.J. Dihydroisocoumarins and a tetralone from Cytospora eucalypticola. Phytochemistry 2003, 62, 779–782. [Google Scholar] [CrossRef]
- Arora, D.; Sharma, N.; Singamaneni, V.; Sharma, V.; Kushwaha, M.; Abrol, V.; Guru, S.; Sharma, S.; Gupta, A.P.; Bhushan, S.; et al. Isolation and characterization of bioactive metabolites from Xylaria psidii, an endophytic fungus of the medicinal plant Aegle marmelos and their role in mitochondrial dependent apoptosis against pancreatic cancer cells. Phytomedicine 2016, 23, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Brian, P.W.; Curtis, P.J.; Howland, S.R.; Jefferys, E.G.; Raudnitz, H. Three new antibiotics from a species of Gliocladium. Experientia 1951, 7, 266–267. [Google Scholar] [CrossRef]
- Steward, M.W.; Packter, N.M. Incorporation of 5-methylorcylaldehyde and methionine into the acetogenin (polyketide) gliorosein in Gliocladium roseum IMI. 93065. Biochem. J. 1968, 109, 1–11. [Google Scholar] [CrossRef]
- Vischer, E.B. The structures of aurantio- and rubro-gliocladin and gliorosein. J. Chem. Soc. 1953, 815–820. [Google Scholar] [CrossRef]
- Muñoz-Sánchez, J.; Chánez-Cárdenas, M.E. The use of cobalt chloride as a chemical hypoxia model. J. Appl. Toxicol. 2019, 39, 556–570. [Google Scholar] [CrossRef]
- Chen, R.; Lai, U.H.; Zhu, L.; Singh, A.; Ahmed, M.; Forsyth, N.R. Reactive oxygen species formation in the brain at different oxygen levels: The role of hypoxia inducible factors. Front. Cell Dev. Biol. 2018, 6, 132. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Li, K.; Ren, Y. Nesfatin-1 protects H9c2 cardiomyocytes against cobalt chloride-induced hypoxic injury by modulating the MAPK and Notch1 signaling pathways. J. Biol. Res. Thessalon. 2021, 28, 21. [Google Scholar] [CrossRef]
- Segura-Aguilar, J. Neurotoxins as Preclinical Models for Parkinson’s Disease. Neurotox. Res. 2018, 34, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Figtree, G.; Aiqun, M.; Ping, Z. GAPDH-knockdown reduce rotenone-induced H9C2 cells death via autophagy and anti-oxidative stress pathway. Toxicol. Lett. 2015, 234, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Yaakoub, H.; Mina, S.; Calenda, A.; Bouchara, J.P.; Papon, N. Oxidative stress response pathways in fungi. Cell. Mol. Life Sci. 2022, 79, 333. [Google Scholar] [CrossRef]
- Yurchenko, E.A.; Menchinskaya, E.S.; Pislyagin, E.A.; Trinh, P.T.H.; Ivanets, E.V.; Smetanina, O.F.; Yurchenko, A.N. Neuroprotective Activity of Some Marine Fungal Metabolites in the 6-Hydroxydopamin- and Paraquat-Induced Parkinson’s Disease Models. Mar. Drugs 2018, 16, 457. [Google Scholar] [CrossRef] [PubMed]







| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC, mult | δH (J in Hz) | δC, mult | δH (J in Hz) | |
| 1 | 196.4, C | 194.9, C | ||
| 2 | 134.7, C | 135.8, C | ||
| 3 | 160.6, C | 159.5, C | ||
| 4 | 72.9, CH | 4.14, d (6.4) | 71.9, CH | 4.25, d (7.4) |
| 5 | 72.8, C | 73.7, C | ||
| 6 | 51.4, CH | 2.20, q (7.4) | 49.1, CH | 2.30, q (6.9) |
| 7 | 20.6, CH3 | 1.11, s | 24.1, CH3 | 1.19, s |
| 8 | 12.9, CH3 | 1.08, d (7.4) | 8.6, CH3 | 1.05, d (6.9) |
| 9 | 57.6, CH3 | 3.92, brs | 58.4, CH3 | 3.93, s |
| 10 | 59.3, CH3 | 3.47, brs | 59.4, CH3 | 3.48, s |
| 4-OH | 5.74, d (6.4) | 5.32, d (7.4) | ||
| 5-OH | 4.76, brs | 4.26, s | ||
| Position | 8 | 9 | 10 | |||
|---|---|---|---|---|---|---|
| δC, mult | δH (J in Hz) | δC, mult | δH (J in Hz) | δC, mult | δH (J в Гц) | |
| 1 | 70.3, CH | 3.79, ddd (12.5, 11.2, 4.7) | 74.1, CH | 5.05, ddd (12.0, 10.0, 4.9) | 67.6, CH | 4.02, ddd (11.7, 10.0, 4.9) |
| 2 | 78.4, CH | 3.24, dd (9.4; 2.8) | 75.5, CH | 3.49, dd (9.8, 2.8) | 81.1, CH | 4.53, dd (9.9, 2.7) |
| 3 | 71.7, CH | 4.15, t (2.4) | 71.6, CH | 4.20, t (2.8) | 68.9, CH | 4.27, t (2.7) |
| 4 | 48.3, CH | 1.42, m | 48.1, CH | 1.45, m | 48.0, CH | 1.49, m |
| 5 | 37.5, CH | 2.36, tdd (12.5, 9.4, 4.0) | 37.2, CH | 2.40, tdd (12.5, 9.0, 4.2) | 37.2, CH | 2.36, tdd (12.5, 9.4, 4.0) |
| 6 | 40.9, CH2 | 1.83, ddd (12.5, 4.7, 4.0) 1.25, q (12.5) | 37.6, CH2 | a: 1.89, dt (12.5, 4.5) b: 1.29, q (12.5) | 40.9, CH2 | a: 1.91, ddd (12.5, 4.9, 4.0) b: 1.33, q (12.5) |
| 7 | 62.4, CH2 | 3.57, d (6.5) | 62.2, CH2 | 3.59, m | 62.0, CH2 | 3.55, m |
| 8 | 172.9, C | 172.8, C | ||||
| 9 | 21.2, CH3 | 2.05, s | 21.1, CH3 | 2.12, s | ||
| 1′ | 137.0, CH | 5.43, dd (14.2, 9.0) | 136.2, CH | 5.42, dd (14.1, 9.0) | 136.4, CH | 5.44, dd (14.1, 9.0) |
| 2′ | 131.3, CH | 6.03–6.13, m | 131.1, CH | 6.05–6.13, m | 131.1, CH | 6.05–6.13, m |
| 3′ | 133.1, CH | 6.03–6.13, m | 133.1, CH | 6.05–6.13, m | 133.1, CH | 6.05–6.13, m |
| 4′ | 132.9, CH | 6.03–6.13, m | 133.0, CH | 6.05–6.13, m | 133.0, CH | 6.05–6.13, m |
| 5′ | 132.3, CH | 6.03–6.13, m | 132.7, CH | 6.05–6.13, m | 132.5, CH | 6.05–6.13, m |
| 6′ | 129.9, CH | 5.68, dq (14.2, 6.9) | 130.0, CH | 5.69, dq (14.2, 6.9) | 130.0, CH | 5.68, dq (14.1, 6.9) |
| 7′ | 18.3, CH3 | 1.75, d (6.9) | 18.3, CH3 | 1.75, d (6.9) | 18.3, CH3 | 1.75, d (6.8) |
| Compound | DPPH Radicals, | IC50, µM | |
|---|---|---|---|
| % of Control 1 | PC-3 | H9c2 | |
| 1 | 93.8 ± 0.5 | >100 | >100 |
| 2 | 100.2 ± 1.0 | >100 | >100 |
| 3 | 86.1 ± 1.8 * | 58.9 ± 1.5 | >100 |
| 4 | 98.6 ± 3.4 | 38.9 ± 1.9 | >100 |
| 5 | 98.9 ± 1.4 | >100 | >100 |
| 8 | 99.4 ± 3.3 | >100 | >100 |
| 9 | 102.7 ± 1.8 | >100 | >100 |
| Ascorbic acid | 10.5 ± 3.2 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinh, P.T.H.; Yurchenko, A.N.; Khmel, O.O.; Dieu, T.V.T.; Ngoc, N.T.D.; Girich, E.V.; Menshov, A.S.; Kim, N.Y.; Chingizova, E.A.; Van, T.T.T.; et al. Cytoprotective Polyketides from Sponge-Derived Fungus Lopadostoma pouzarii. Molecules 2022, 27, 7650. https://doi.org/10.3390/molecules27217650
Trinh PTH, Yurchenko AN, Khmel OO, Dieu TVT, Ngoc NTD, Girich EV, Menshov AS, Kim NY, Chingizova EA, Van TTT, et al. Cytoprotective Polyketides from Sponge-Derived Fungus Lopadostoma pouzarii. Molecules. 2022; 27(21):7650. https://doi.org/10.3390/molecules27217650
Chicago/Turabian StyleTrinh, Phan Thi Hoai, Anton N. Yurchenko, Olga O. Khmel, Trang Vo Thi Dieu, Ngo Thi Duy Ngoc, Elena V. Girich, Alexander S. Menshov, Natalya Y. Kim, Ekaterina A. Chingizova, Tran Thi Thanh Van, and et al. 2022. "Cytoprotective Polyketides from Sponge-Derived Fungus Lopadostoma pouzarii" Molecules 27, no. 21: 7650. https://doi.org/10.3390/molecules27217650
APA StyleTrinh, P. T. H., Yurchenko, A. N., Khmel, O. O., Dieu, T. V. T., Ngoc, N. T. D., Girich, E. V., Menshov, A. S., Kim, N. Y., Chingizova, E. A., Van, T. T. T., Lee, J. S., Lee, H. -S., & Yurchenko, E. A. (2022). Cytoprotective Polyketides from Sponge-Derived Fungus Lopadostoma pouzarii. Molecules, 27(21), 7650. https://doi.org/10.3390/molecules27217650