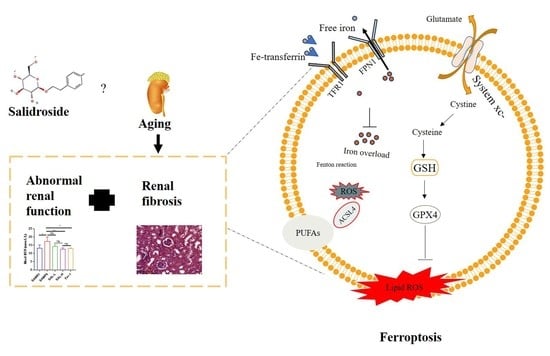
Salidroside Alleviates Renal Fibrosis in SAMP8 Mice by Inhibiting Ferroptosis
Abstract
:1. Introduction
2. Results
2.1. Screened Potential Targets
2.2. Enrichment Analysis of Potential Targets
2.3. Network Analysis of Potential Targets
2.4. Effects of SAL Treatment on Renal Function Parameters from the Serum of SAMP8 Mice
2.5. Effects of SAL Treatment on Histopathological Changes in the Kidneys of SAMP8 Mice
2.6. SAL Ameliorates Renal Interstitial Fibrosis in SAMP8 Mice
2.7. Effects of SAL Treatment in SAMP8 Mice on Lipid Peroxidation and GPX4
2.8. SAL Ameliorates Renal Iron Overload in SAMP8 Mice
2.9. SAL Ameliorates Renal Ferroptosis in SAMP8 Mice
2.10. Molecular Docking Analysis
3. Discussion
4. Materials and Methods
4.1. Screen Targets of SAL and Aging-Related Renal Fibrosis
4.2. Enrichment Analysis and Network Construction
4.3. Experimental Design
4.4. Biochemical Analysis
4.5. Histology and Morphometry
4.6. Kidney Iron Staining
4.7. Measurement of Malondialdehyde (MDA), Superoxide Dismutase (SOD), and Glutathione (GSH)
4.8. Polymerase Chain Reaction
4.9. Western Blotting
4.10. Molecular Docking
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the global challenges of ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Bowe, B.; Mokdad, A.H.; Xian, H.; Yan, Y.; Li, T.; Maddukuri, G.; Tsai, C.Y.; Floyd, T.; Al-Aly, Z. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018, 94, 567–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Sullivan, E.-D.; Hughes, J.; Ferenbach, D.-A. Renal Aging: Causes and Consequences. J. Am. Soc. Nephrol. 2017, 28, 407–420. [Google Scholar] [CrossRef] [Green Version]
- Glassock, R.J.; Warnock, D.G.; Delanaye, P. The global burden of chronic kidney disease: Estimates, variability and pit-falls. Nat. Rev. Nephrol. 2017, 13, 104–114. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Takeda, T. Senescence-accelerated mouse (SAM) with special references to neurodegeneration models, SAMP8 and SAMP10 mice. Neurochem. Res. 2009, 34, 639–659. [Google Scholar] [CrossRef]
- Takeda, T. Senescence-accelerated mouse (SAM): With special reference to age-associated pathologies and their modulation. Nihon Eiseigaku Zasshi 1996, 51, 569–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, T.; Matsushita, T.; Kurozumi, M.; Takemura, K.; Higuchi, K.; Hosokawa, M. Pathobiology of the senes-cence-accelerated mouse (SAM). Exp. Gerontol. 1997, 32, 117–127. [Google Scholar] [CrossRef]
- Taniguchi, S.; Hanafusa, M.; Tsubone, H.; Takimoto, H.; Yamanaka, D.; Kuwahara, M.; Ito, K. Age-dependency of the serum oxidative level in the senescence-accelerated mouse prone 8. J. Vet. Med. Sci. 2016, 78, 1369–1371. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, N.; Endo, J.; Kinouchi, K.; Kitakata, H.; Moriyama, H.; Kataoka, M.; Yamamoto, T.; Shirakawa, K.; Morimoto, S.; Nishiyama, A.; et al. (Pro)renin receptor accelerates develop-ment of sarcopenia via activation of Wnt/YAP signaling axis. Aging Cell 2019, 18, e12991. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Arumugam, S.; Babu, S.S.; Palaniyandi, S.S.; Watanabe, K.; Cooke, J.P.; Thandavarayan, R. The senescence accelerated mouse prone 8 (SAMP8): A novel murine model for cardiac aging. Ageing Res. Rev. 2017, 35, 291–296. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Li, L.; Han, Y.; Dong, X.; Yang, L.; Li, X.; Li, W.; Li, W. Ginsenoside Rg1 ameliorates aging-induced liver fibrosis by in-hibiting the NOX4/NLRP3 inflammasome in SAMP8 mice. Mol. Med. Rep. 2021, 24, 801. [Google Scholar] [CrossRef]
- Shen, X.; Dong, X.; Han, Y.; Li, Y.; Ding, S.; Zhang, H.; Sun, Z.; Yin, Y.; Li, W.; Li, W. Ginsenoside Rg1 ameliorates glomerular fibrosis during kidney aging by inhibiting NOX4 and NLRP3 inflammasome activation in SAMP8 mice. Int. Immunopharmacol. 2020, 82, 106339. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Liu, J.; Niu, J.; Zhang, Y.; Shen, W.; Luo, C.; Liu, Y.; Li, C.; Li, H.; Yang, P.; et al. Wnt/β-catenin/RAS signaling mediates age-related renal fibrosis and is associated with mitochondrial dysfunction. Aging Cell 2019, 18, e13004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakahara, H.; Kanno, T.; Inai, Y.; Utsumi, K.; Hiramatsu, M.; Mori, A.; Packer, L. Mitochondrial dysfunction in the senescence accelerated mouse (SAM). Free. Radic. Biol. Med. 1998, 24, 85–92. [Google Scholar] [CrossRef]
- Cao, B.; Zeng, M.; Si, Y.; Zhang, B.; Wang, Y.; Xu, R.; Huang, Y.; Feng, W.; Zheng, X. Extract of Corallodiscus flabellata attenuates renal fibrosis in SAMP8 mice via the Wnt/β-catenin/RAS signaling pathway. BMC Complement. Med. Ther. 2022, 22, 52. [Google Scholar] [CrossRef]
- Baltanás, A.; Solesio, M.E.; Zalba, G.; Galindo, M.F.; Fortuño, A.; Jordán, J. The senescence-accelerated mouse prone-8 (SAM-P8) oxidative stress is associated with upregulation of renal NADPH oxidase system. J. Physiol. Biochem. 2013, 69, 927–935. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, P.H.; Zhang, M.; Du, J.R. Aging-related renal injury and inflammation are associated with downregulation of Klotho and induction of RIG-I/NF-κB signaling pathway in senescence-accelerated mice. Aging Clin. Exp. Res. 2016, 28, 69–76. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, B.; Shen, D.; Chen, J.; Yu, Z.; Chen, C. Ferroptosis in a sarcopenia model of senescence accelerated mouse prone 8 (SAMP8). Int. J. Biol. Sci. 2021, 17, 151–162. [Google Scholar] [CrossRef]
- Belavgeni, A.; Meyer, C.; Stumpf, J.; Hugo, C.; Linkermann, A. Ferroptosis and Necroptosis in the Kidney. Cell Chem. Biol. 2020, 27, 448–462. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, X.; Yi, X.; Zhu, X.H.; Jiang, D.S. Posttranslational Modifications in Ferroptosis. Oxid. Med. Cell. Longev. 2020, 2020, 8832043. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kroemer, G. Ferroptosis. Curr. Biol. 2020, 30, R1292–R1297. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Aslkhodapasandhokmabad, H.; Libby, P.; Tuomilehto, J.; Lip, G.Y.; Penninger, J.M.; Richardson, D.R.; Tang, D.; Zhou, H.; Wang, S.; et al. Ferritinophagy and ferroptosis in the management of metabolic diseases. Trends Endocrinol. Metab. 2021, 32, 444–462. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, A.R.; Miyazawa, M.; Hashimoto, K.; Tsuji, Y. Regulators of Iron Homeostasis: New Players in Metabolism, Cell Death, and Disease. Trends Biochem. Sci. 2016, 41, 274–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, F.; Yang, L.; Li, R.; Zou, X.; Li, N.; Meng, X.; Zhang, Y.; Wang, X. Salidroside as a potential neuroprotective agent for ischemic stroke: A review of sources, pharmacokinetics, mechanism and safety. Biomed. Pharmacother. 2020, 129, 110458. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, J.D.; Panossian, A.G. Rhodiola rosea L. as a putative botanical antidepressant. Phytomedicine 2016, 23, 770–783. [Google Scholar] [CrossRef]
- Li, R.; Chen, J. Salidroside Protects Dopaminergic Neurons by Enhancing PINK1/Parkin-Mediated Mitophagy. Oxid. Med. Cell. Longev. 2019, 2019, 9341018. [Google Scholar] [CrossRef] [Green Version]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef] [Green Version]
- Humphreys, B.D. Mechanisms of Renal Fibrosis. Annu. Rev. Physiol. 2018, 80, 309–326. [Google Scholar] [CrossRef]
- Jin, H.; Pei, L.; Shu, X.; Yang, X.; Yan, T.; Wu, Y.; Wei, N.; Yan, H.; Wang, S.; Yao, C.; et al. Therapeutic Intervention of Learning and Memory Decays by Salidroside Stimulation of Neurogenesis in Aging. Mol. Neurobiol. 2016, 53, 851–866. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, J.; Le, Y.; Pan, J.; Liu, Y.; Liu, Z.; Wang, C.; Dou, X.; Lu, D. Salidroside inhibits doxorubicin-induced cardiomyopathy by modulating a ferroptosis-dependent pathway. Phytomedicine 2022, 99, 153964. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.J.; Huber, T.B.; Isermann, B.; Schiffer, M. CKD in diabetes: Diabetic kidney disease versus nondiabetic kidney disease. Nat. Rev. Nephrol. 2018, 14, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Luo, C.; Peng, F.; Xiao, J.; Zeng, Y.; Yin, B.; Chen, X.; Li, S.; He, X.; Liu, Y.; et al. Brahma-related gene-1 promotes tubular senescence and renal fibrosis through Wnt/beta-catenin/autophagy axis. Clin. Sci. 2021, 135, 1873–1895. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Li, Y.; Xu, Y.; Guo, R. Salidroside protects against cardiomyocyte apoptosis and ventricular remodeling by AKT/HO-1 signaling pathways in a diabetic cardiomyopathy mouse model. Phytomedicine 2021, 82, 153406. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Guo, Y.; Zhang, Y.; Zhang, X.; Zhu, L.; Yan, T. Salidroside Ameliorates Renal Interstitial Fibrosis by Inhibiting the TLR4/NF-kappa B and MAPK Signaling Pathways. Int. J. Mol. Sci. 2019, 20, 1103. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, Z.Y.; Zheng, J.H.; Li, S. TCM network pharmacology: A new trend towards combining computational, experimental and clinical approaches. Chin. J. Nat. Med. 2021, 19, 1–11. [Google Scholar] [CrossRef]
- Bai, T.; Lei, P.; Zhou, H.; Liang, R.; Zhu, R.; Wang, W.; Zhou, L.; Sun, Y. Sigma-1 receptor protects against ferroptosis in hepatocellular carcinoma cells. J. Cell Mol. Med. 2019, 23, 7349–7359. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Kang, S.W.; Joo, J.; Han, S.H.; Shin, H.; Nam, B.Y.; Park, J.; Yoo, T.H.; Kim, G.; Lee, P.; et al. Characterization of ferroptosis in kidney tubular cell death under diabetic conditions. Cell Death Dis. 2021, 12, 160. [Google Scholar] [CrossRef]
- Tan, H.; Chen, J.; Li, Y.; Li, Y.; Zhong, Y.; Li, G.; Liu, L.; Li, Y. Glabridin, a bioactive component of licorice, ameliorates diabetic nephropathy by regulating ferroptosis and the VEGF/Akt/ERK pathways. Mol. Med. 2022, 28, 58. [Google Scholar] [CrossRef]
- Fang, X.; Cai, Z.; Wang, H.; Han, D.; Cheng, Q.; Zhang, P.; Gao, F.; Yu, Y.; Song, Z.; Wu, Q.; et al. Loss of Cardiac Ferritin H Facilitates Cardiomyopathy via Slc7a11-Mediated Ferroptosis. Circ. Res. 2020, 127, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Sha, R.; Xu, Y.; Yuan, C.; Sheng, X.; Wu, Z.; Peng, J.; Wang, Y.; Lin, Y.; Zhou, L.; Xu, S.; et al. Predictive and prognostic impact of ferroptosis-related genes ACSL4 and GPX4 on breast cancer treated with neoadjuvant chemotherapy. Ebiomedicine 2021, 71, 103560. [Google Scholar] [CrossRef] [PubMed]
- Weigand, I.; Schreiner, J.; Rohrig, F.; Sun, N.; Landwehr, L.S.; Urlaub, H.; Kendl, S.; Kiseljak-Vassiliades, K.; Wierman, M.E.; Angeli, J.P.F.; et al. Active steroid hormone synthesis renders adrenocortical cells highly susceptible to type II ferroptosis induction. Cell Death Dis. 2020, 11, 192. [Google Scholar] [CrossRef] [Green Version]
- Lei, G.; Zhang, Y.; Koppula, P.; Liu, X.; Zhang, J.; Lin, S.H.; Ajani, J.A.; Xiao, Q.; Liao, Z.; Wang, H.; et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020, 30, 146–162. [Google Scholar] [CrossRef] [PubMed]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Xie, Z.; Pei, T.; Zeng, Y.; Xiong, Q.; Wei, H.; Wang, Y.; Cheng, W. Salidroside attenuates neuronal ferroptosis by activating the Nrf2/HO1 signaling pathway in Aβ1-42-induced Alzheimer’s disease mice and glutamate-injured HT22 cells. Chin. Med. 2022, 17, 82. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]










| Uniprot ID | PDB ID | AlphaFord ID | Gene Symbol |
|---|---|---|---|
| P36970 | 5l71 | GPX4 | |
| D4ADU2 | Q9WTR6 | SLC7A11 | |
| O35547 | Q9QUJ7 | ACSL4 | |
| P19132 | 5xb1 | FTH1 | |
| Q99376 | Q62351 | TFR1 | |
| Q923U9 | Q9JHI9 | FPN1 |
| GPX4 | Forward primer | CCGCCGAGATGAGCTGG |
| Reverse primer | GTCGATGTCCTTGGCTGAG | |
| SLC7A11 | Forward primer | ATGGTCAGAAAGCCAGTTGTG |
| Reverse primer | CAGGGCGTATTACGAGCAGT | |
| ACSL4 | Forward primer | TCCCTGGACTAGGACCGAAG |
| Reverse primer | GGGGCGTCATAGCCTTTCTT | |
| TGF-β | Forward primer | CCAGATCCTGTCCAAACTAAGG |
| Reverse primer | CTCTTTAGCATAGTAGTCCGCT | |
| α-sma | Forward primer | CTATGAGGGCTATGCCTTGCC |
| Reverse primer | GCTCAGCAGTAGTAACGAAGGA | |
| β-actin | Forward primer | CCACCATGTACCCAGGCATT |
| Reverse primer | CGGACTCATCGTACTCCTGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Pei, T.; Wang, L.; Zeng, Y.; Li, W.; Yan, S.; Xiao, W.; Cheng, W. Salidroside Alleviates Renal Fibrosis in SAMP8 Mice by Inhibiting Ferroptosis. Molecules 2022, 27, 8039. https://doi.org/10.3390/molecules27228039
Yang S, Pei T, Wang L, Zeng Y, Li W, Yan S, Xiao W, Cheng W. Salidroside Alleviates Renal Fibrosis in SAMP8 Mice by Inhibiting Ferroptosis. Molecules. 2022; 27(22):8039. https://doi.org/10.3390/molecules27228039
Chicago/Turabian StyleYang, Sixia, Tingting Pei, Linshuang Wang, Yi Zeng, Wenxu Li, Shihua Yan, Wei Xiao, and Weidong Cheng. 2022. "Salidroside Alleviates Renal Fibrosis in SAMP8 Mice by Inhibiting Ferroptosis" Molecules 27, no. 22: 8039. https://doi.org/10.3390/molecules27228039
APA StyleYang, S., Pei, T., Wang, L., Zeng, Y., Li, W., Yan, S., Xiao, W., & Cheng, W. (2022). Salidroside Alleviates Renal Fibrosis in SAMP8 Mice by Inhibiting Ferroptosis. Molecules, 27(22), 8039. https://doi.org/10.3390/molecules27228039