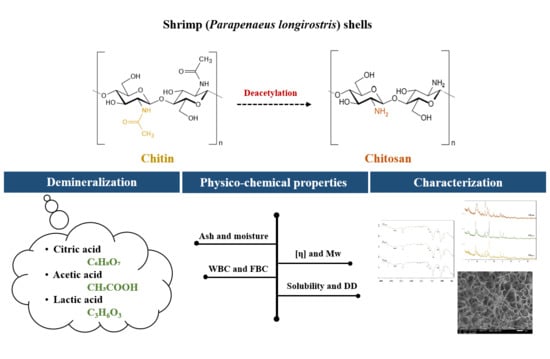
Physicochemical Properties and Functional Characteristics of Ecologically Extracted Shrimp Chitosans with Different Organic Acids during Demineralization Step
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction of Chitosan Samples from Shrimp Shells
2.1.1. Deproteinization Step
2.1.2. Demineralization Step
2.1.3. Deacetylation Step
2.2. Physicochemical Properties of Chitosan Samples
2.2.1. Ash and Moisture Contents
2.2.2. Water Binding Capacity (WBC) and Fat Binding Capacity (FBC)
2.2.3. Solubility Determination
- WInitial is the initial weight of tube;
- WInitial+S and WFinal+S are the initial and the final weight of tube + sample, respectively.
2.2.4. Degree of Deacetylation (DD) Determination
- A1655 represents absorption degree at 1655 cm−1;
- A3450 represents absorption degree at 3450 cm−1.
2.2.5. Molecular Weight (Mw) Determination
2.3. Characterization of Chitosan Samples
2.3.1. Fourier Transform Infrared (FTIR) Spectroscopy
2.3.2. X-ray Diffraction (XRD)
- Iam is amorphous diffraction intensity at 2θ;
- I110 is crystalline material maximum intensity at 2θ.
2.3.3. Scanning Electron Microscopy (SEM)
2.4. Statistical Analyses
3. Results and Discussion
3.1. Extraction of Chitosan Samples from Shrimp Shells
3.2. Physicochemical Properties of Chitosan Samples
3.2.1. Ash and Moisture Contents
3.2.2. Water Binding Capacity (WBC) and Fat Binding Capacity (FBC)
3.2.3. Solubility Determination
3.2.4. Degree of Deacetylation (DD) Determination
3.2.5. Molecular Weight (Mw) Determination
3.3. Characterization of Chitosan Samples
3.3.1. Fourier Transform Infrared (FTIR) Spectroscopy
3.3.2. X-ray Diffraction (XRD)
3.3.3. Scanning Electron Microscopy (SEM)
3.4. Dimensional Analysis of the Resulting Data
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kulawik, P.; Jamróz, E.; Özogul, F. Chitosan Role for Shelf-Life Extension of Seafood. Environ. Chem. Lett. 2020, 18, 61–74. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- McKay, G. Use of Adsorbents for the Removal of Pollutants from Wastewater; CRC Press: Boca Raton, FL, USA, 1995; ISBN 978-0-8493-6920-9. [Google Scholar]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef] [PubMed]
- Yarnpakdee, S.; Kaewprachu, P.; Jaisan, C.; Senphan, T.; Nagarajan, M.; Wangtueai, S. Extraction and Physico–Chemical Characterization of Chitosan from Mantis Shrimp (Oratosquilla nepa) Shell and the Development of Bio-Composite Film with Agarose. Polymers 2022, 14, 3983. [Google Scholar] [CrossRef]
- Ullah, N.; Basit, A.; Ahmad, I.; Ullah, I.; Shah, S.T.; Mohamed, H.I.; Javed, S. Mitigation the Adverse Effect of Salinity Stress on the Performance of the Tomato Crop by Exogenous Application of Chitosan. Bull. Natl. Res. Cent. 2020, 44, 181. [Google Scholar] [CrossRef]
- Hassan, F.A.S.; Ali, E.; Gaber, A.; Fetouh, M.I.; Mazrou, R. Chitosan Nanoparticles Effectively Combat Salinity Stress by Enhancing Antioxidant Activity and Alkaloid Biosynthesis in Catharanthus roseus (L.) G. Don. Plant Physiol. Biochem. 2021, 162, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Anraku, M.; Gebicki, J.M.; Iohara, D.; Tomida, H.; Uekama, K.; Maruyama, T.; Hirayama, F.; Otagiri, M. Antioxidant Activities of Chitosans and Its Derivatives in in Vitro and in Vivo Studies. Carbohydr. Polym. 2018, 199, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Pakdel, M.P.; Peighambardoust, S.J. A Review on Acrylic Based Hydrogels and Their Applications in Wastewater Treatment. J. Environ. Manage. 2018, 217, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M.; Gopal, J.; Chun, S.; Devadoss, A.J.P.; Hasan, N.; Sivanesan, I. Crustacean Waste-Derived Chitosan: Antioxidant Properties and Future Perspective. Antioxidants 2021, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current Advancements in Chitosan-Based Film Production for Food Technology; A Review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z. Pharmaceutical Applications of Chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Pal, A.; Nakashima, K.; Yadav, B.K. Applications of Chitosan in Environmental Remediation: A Review. Chemosphere 2021, 266, 128934. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, M.; Fényes, E.; Bartos, C.; Zeeshan, M.; Ambrus, R. Chitosan Biopolymer, Its Derivatives and Potential Applications in Nano-Therapeutics: A Comprehensive Review. Eur. Polym. J. 2021, 160, 110767. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Bhardwaj, N.K.; Negi, Y.S. Effect of Degree of Deacetylation of Chitosan on Its Performance as Surface Application Chemical for Paper-Based Packaging. Cellulose 2020, 27, 5337–5352. [Google Scholar] [CrossRef]
- Weißpflog, J.; Vehlow, D.; Müller, M.; Kohn, B.; Scheler, U.; Boye, S.; Schwarz, S. Characterization of Chitosan with Different Degree of Deacetylation and Equal Viscosity in Dissolved and Solid State—Insights by Various Complimentary Methods. Int. J. Biol. Macromol. 2021, 171, 242–261. [Google Scholar] [CrossRef] [PubMed]
- Kou, S. (Gabriel); Peters, L.; Mucalo, M. Chitosan: A Review of Molecular Structure, Bioactivities and Interactions with the Human Body and Micro-Organisms. Carbohydr. Polym. 2022, 282, 119132. [Google Scholar] [CrossRef]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural Modification, Biological Activity and Application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef] [PubMed]
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical Extraction and Modification of Chitin and Chitosan from Shrimp Shells. Eur. Polym. J. 2021, 159, 110709. [Google Scholar] [CrossRef]
- Srinivasan, H.; Kanayairam, V.; Ravichandran, R. Chitin and Chitosan Preparation from Shrimp Shells Penaeus monodon and Its Human Ovarian Cancer Cell Line, PA-1. Int. J. Biol. Macromol. 2018, 107, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood Waste: A Source for Preparation of Commercially Employable Chitin/Chitosan Materials. Bioresour. Bioprocess. 2019, 6, 8. [Google Scholar] [CrossRef]
- Trung, T.S.; Tram, L.H.; Van Tan, N.; Van Hoa, N.; Minh, N.C.; Loc, P.T.; Stevens, W.F. Improved Method for Production of Chitin and Chitosan from Shrimp Shells. Carbohydr. Res. 2020, 489, 107913. [Google Scholar] [CrossRef] [PubMed]
- Leo Edward, M.; Dharanibalaji, K.C.; Kumar, K.T.; Chandrabose, A.R.S.; Shanmugharaj, A.M.; Jaisankar, V. Preparation and Characterisation of Chitosan Extracted from Shrimp Shell (Penaeus monodon) and Chitosan-Based Blended Solid Polymer Electrolyte for Lithium-Ion Batteries. Polym. Bull. 2022, 79, 587–604. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, Chemical Modification and Characterization of Chitin and Chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Tolesa, L.D.; Gupta, B.S.; Lee, M.-J. Chitin and Chitosan Production from Shrimp Shells Using Ammonium-Based Ionic Liquids. Int. J. Biol. Macromol. 2019, 130, 818–826. [Google Scholar] [CrossRef]
- El-araby, A.; El Ghadraoui, L.; Errachidi, F. Usage of Biological Chitosan against the Contamination of Post-Harvest Treatment of Strawberries by Aspergillus Niger. Front. Sustain. Food Syst. 2022, 6, 881434. [Google Scholar] [CrossRef]
- Lertsutthiwong, P.; Chandrkrachang, S.; Stevens, W.F. The Effect of the Utilization of Chitosan on Properties of Paper. J. Met. Mater. Miner. 2000, 10, 43–52. [Google Scholar]
- Hossain, M.; Iqbal, A. Production and Characterization of Chitosan from Shrimp Waste. J. Bangladesh Agric. Univ. 2014, 12, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, S.; Bhardwaj, N.K.; Negi, Y.S. Cleaner Approach for Improving the Papermaking from Agro and Hardwood Blended Pulps Using Biopolymers. J. Clean. Prod. 2019, 213, 134–142. [Google Scholar] [CrossRef]
- Wang, J.C.; Kinsella, J.E. Functional Properties of Novel Proteins: Alfalfa Leaf Protein. J. Food Sci. 1976, 41, 286–292. [Google Scholar] [CrossRef]
- Baxter, A.; Dillon, M.; Anthony Taylor, K.D.; Roberts, G.A.F. Improved Method for i.r. Determination of the Degree of N-Acetylation of Chitosan. Int. J. Biol. Macromol. 1992, 14, 166–169. [Google Scholar] [CrossRef]
- Domard, A.; Rinaudo, M. Preparation and Characterization of Fully Deacetylated Chitosan. Int. J. Biol. Macromol. 1983, 5, 49–52. [Google Scholar] [CrossRef]
- Czechowska-Biskup, R.; Jarosińska, D.; Rokita, B.; Ulański, P.; Rosiak, J.M. Determination of degree of deacetylation of chitosan-comparision of methods. Prog. Chem. Appl. Chitin Its Deriv. 2012, 17, 5–20. [Google Scholar]
- Jiang, Y.; Fu, C.; Wu, S.; Liu, G.; Guo, J.; Su, Z. Determination of the Deacetylation Degree of Chitooligosaccharides. Mar. Drugs 2017, 15, 332. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M.; Milas, M.; Dung, P.L. Characterization of Chitosan. Influence of Ionic Strength and Degree of Acetylation on Chain Expansion. Int. J. Biol. Macromol. 1993, 15, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.A.F.; Domszy, J.G. Determination of the Viscometric Constants for Chitosan. Int. J. Biol. Macromol. 1982, 4, 374–377. [Google Scholar] [CrossRef]
- Kasaai, M.R.; Arul, J.; Charlet, G. Intrinsic Viscosity-Molecular Weight Relationship for Chitosan. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 2591–2598. [Google Scholar] [CrossRef]
- Focher, B.; Beltrame, P.L.; Naggi, A.; Torri, G. Alkaline N-Deacetylation of Chitin Enhanced by Flash Treatments. Reaction Kinetics and Structure Modifications. Carbohydr. Polym. 1990, 12, 405–418. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a Bioactive Polymer: Processing, Properties and Applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Kou, S. (Gabriel); Peters, L.M.; Mucalo, M.R. Chitosan: A Review of Sources and Preparation Methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Amoo, K.; Olafadehan, A.O.; Ajayi, O.T. Optimization Studies of Chitin and Chitosan Production from Penaeus notialis Shell Waste. Afr. J. Biotechnol. 2019, 18, 670–688. [Google Scholar] [CrossRef]
- Olaosebikan, A.O.; Kehinde, O.A.; Tolulase, O.A.; Victor, E.B. Extraction and Characterization of Chitin and Chitosan from Callinectes amnicola and Penaeus notialis Shell Wastes. J. Chem. Eng. Mater. Sci. 2021, 12, 1–30. [Google Scholar] [CrossRef]
- Rakkhumkaew, N.; Pengsuk, C. Chitosan and Chitooligosaccharides from Shrimp Shell Waste: Characterization, Antimicrobial and Shelf Life Extension in Bread. Food Sci. Biotechnol. 2018, 27, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Muley, A.B.; Chaudhari, S.A.; Mulchandani, K.H.; Singhal, R.S. Extraction and Characterization of Chitosan from Prawn Shell Waste and Its Conjugation with Cutinase for Enhanced Thermo-Stability. Int. J. Biol. Macromol. 2018, 111, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Wang, Y.; Han, Q.; Ji, L.; Zhang, H.; Fei, Z.; Wang, Y. Comparison of the Physicochemical, Rheological, and Morphologic Properties of Chitosan from Four Insects. Carbohydr. Polym. 2019, 209, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Núñez, J.R.; Madera-Santana, T.J.; Sánchez-Machado, D.I.; López-Cervantes, J.; Soto Valdez, H. Chitosan/Hydrophilic Plasticizer-Based Films: Preparation, Physicochemical and Antimicrobial Properties. J. Polym. Environ. 2014, 22, 41–51. [Google Scholar] [CrossRef]
- Baron, R.D.; Pérez, L.L.; Salcedo, J.M.; Córdoba, L.P.; Sobral, P.J.d.A. Production and Characterization of Films Based on Blends of Chitosan from Blue Crab (Callinectes sapidus) Waste and Pectin from Orange (Citrus sinensis Osbeck) Peel. Int. J. Biol. Macromol. 2017, 98, 676–683. [Google Scholar] [CrossRef]
- No, H.K.; Meyers, S.P. Preparation and Characterization of Chitin and Chitosan—A Review. J. Aquat. Food Prod. Technol. 1995, 4, 27–52. [Google Scholar] [CrossRef]
- Martínez-Camacho, A.P.; Cortez-Rocha, M.O.; Ezquerra-Brauer, J.M.; Graciano-Verdugo, A.Z.; Rodriguez-Félix, F.; Castillo-Ortega, M.M.; Yépiz-Gómez, M.S.; Plascencia-Jatomea, M. Chitosan Composite Films: Thermal, Structural, Mechanical and Antifungal Properties. Carbohydr. Polym. 2010, 82, 305–315. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar Annamareddy, S.H.; Abanti, S.; Kumar Rath, P. Physicochemical Properties and Characterization of Chitosan Synthesized from Fish Scales, Crab and Shrimp Shells. Int. J. Biol. Macromol. 2017, 104, 1697–1705. [Google Scholar] [CrossRef]
- Al-Manhel, A.J.; Al-Hilphy, A.R.S.; Niamah, A.K. Extraction of Chitosan, Characterisation and Its Use for Water Purification. J. Saudi Soc. Agric. Sci. 2018, 17, 186–190. [Google Scholar] [CrossRef] [Green Version]
- Ocloo, F.C.K.; Quayson, E.T.; Adu-Gyamfi, A.; Quarcoo, E.A.; Asare, D.; Serfor-Armah, Y.; Woode, B.K. Physicochemical and Functional Characteristics of Radiation-Processed Shrimp Chitosan. Radiat. Phys. Chem. 2011, 80, 837–841. [Google Scholar] [CrossRef]
- Cho, Y.I.; No, H.K.; Meyers, S.P. Physicochemical Characteristics and Functional Properties of Various Commercial Chitin and Chitosan Products. J. Agric. Food Chem. 1998, 46, 3839–3843. [Google Scholar] [CrossRef]
- Chien, R.-C.; Yen, M.-T.; Mau, J.-L. Antimicrobial and Antitumor Activities of Chitosan from Shiitake Stipes, Compared to Commercial Chitosan from Crab Shells. Carbohydr. Polym. 2016, 138, 259–264. [Google Scholar] [CrossRef]
- Samar, M.M.; El-Kalyoubi, M.H.; Khalaf, M.M.; Abd El-Razik, M.M. Physicochemical, Functional, Antioxidant and Antibacterial Properties of Chitosan Extracted from Shrimp Wastes by Microwave Technique. Ann. Agric. Sci. 2013, 58, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and Antioxidant Properties of Chitosan and Its Derivatives and Their Applications: A Review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef] [PubMed]
- Metin, C.; Alparslan, Y.; Baygar, T.; Baygar, T. Physicochemical, Microstructural and Thermal Characterization of Chitosan from Blue Crab Shell Waste and Its Bioactivity Characteristics. J. Polym. Environ. 2019, 27, 2552–2561. [Google Scholar] [CrossRef]
- Pérez-Álvarez, L.; Ruiz-Rubio, L.; Vilas-Vilela, J.L. Determining the Deacetylation Degree of Chitosan: Opportunities to Learn Instrumental Techniques. J. Chem. Educ. 2018, 95, 1022–1028. [Google Scholar] [CrossRef]
- Kuyyogsuy, A. Preparation and Characterization of Chitosan Obtained FromPacific White Shrimp Shells and its in Vitro Antifungal Activity. Asian J. Chem. 2020, 32, 2515–2519. [Google Scholar] [CrossRef]
- John Kasongo, K.; Tubadi, D.J.; Bampole, L.D.; Kaniki, T.A.; Kanda, N.J.M.; Lukumu, M.E. Extraction and Characterization of Chitin and Chitosan from Termitomyces titanicus. SN Appl. Sci. 2020, 2, 406. [Google Scholar] [CrossRef] [Green Version]
- No, H.K.; Hur, E.Y. Control of Foam Formation by Antifoam during Demineralization of Crustacean Shell in Preparation of Chitin. J. Agric. Food Chem. 1998, 46, 3844–3846. [Google Scholar] [CrossRef]
- Aldila, H.; Asmar; Fabiani, V.A.; Dalimunthe, D.Y.; Irwanto, R. The Effect of Deproteinization Temperature and NaOH Concentration on Deacetylation Step in Optimizing Extraction of Chitosan from Shrimp Shells Waste. IOP Conf. Ser. Earth Environ. Sci. 2020, 599, 012003. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as A Preservative for Fruits and Vegetables: A Review on Chemistry and Antimicrobial Properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar] [CrossRef]
- Czechowska-Biskup, R.; Wach, R.A.; Rosiak, J.M.; Ulański, P. Procedure for determination of the molecular weight of chitosan by viscometry. Prog. Chem. Appl. Chitin Its Deriv. 2018, 23, 45–54. [Google Scholar] [CrossRef]
- Acosta-Ferreira, S.; Castillo, O.S.; Madera-Santana, J.T.; Mendoza-García, D.A.; Núñez-Colín, C.A.; Grijalva-Verdugo, C.; Villa-Lerma, A.G.; Morales-Vargas, A.T.; Rodríguez-Núñez, J.R. Production and Physicochemical Characterization of Chitosan for the Harvesting of Wild Microalgae Consortia. Biotechnol. Rep. 2020, 28, e00554. [Google Scholar] [CrossRef] [PubMed]
- de Farias, B.S.; Grundmann, D.D.R.; Rizzi, F.Z.; Martins, N.S.S.; Sant’Anna Cadaval Junior, T.R.; de Almeida Pinto, L.A. Production of Low Molecular Weight Chitosan by Acid and Oxidative Pathways: Effect on Physicochemical Properties. Food Res. Int. 2019, 123, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yuan, Y.; Duan, S.; Li, C.; Hu, B.; Liu, A.; Wu, D.; Cui, H.; Lin, L.; He, J.; et al. Preparation and Characterization of Chitosan Films with Three Kinds of Molecular Weight for Food Packaging. Int. J. Biol. Macromol. 2020, 155, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Shigemasa, Y.; Matsuura, H.; Sashiwa, H.; Saimoto, H. Evaluation of Different Absorbance Ratios from Infrared Spectroscopy for Analyzing the Degree of Deacetylation in Chitin. Int. J. Biol. Macromol. 1996, 18, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.L.; Ferreira, M.C.; Marvão, M.R.; Rocha, J. An Optimised Method to Determine the Degree of Acetylation of Chitin and Chitosan by FTIR Spectroscopy. Int. J. Biol. Macromol. 2002, 31, 1–8. [Google Scholar] [CrossRef]
- Koc, B.; Akyuz, L.; Cakmak, Y.S.; Sargin, I.; Salaberria, A.M.; Labidi, J.; Ilk, S.; Cekic, F.O.; Akata, I.; Kaya, M. Production and Characterization of Chitosan-Fungal Extract Films. Food Biosci. 2020, 35, 100545. [Google Scholar] [CrossRef]
- Varma, R.; Vasudevan, S. Extraction, Characterization, and Antimicrobial Activity of Chitosan from Horse Mussel Modiolus Modiolus. ACS Omega 2020, 5, 20224–20230. [Google Scholar] [CrossRef] [PubMed]
- Mourya, V.K.; Inamdar, N.N.; Choudhari, Y.M. Chitooligosaccharides: Synthesis, Characterization and Applications. Polym. Sci. Ser. A 2011, 53, 583–612. [Google Scholar] [CrossRef]
- Panda, P.K.; Dash, P.; Chang, Y.-H.; Yang, J.-M. Improvement of Chitosan Water Solubility by Fumaric Acid Modification. Mater. Lett. 2022, 316, 132046. [Google Scholar] [CrossRef]
- Rasti, H.; Parivar, K.; Baharara, J.; Iranshahi, M.; Namvar, F. Chitin from the Mollusc Chiton: Extraction, Characterization and Chitosan Preparation. Iran. J. Pharm. Res. 2017, 16, 366. [Google Scholar] [PubMed]
- Ibitoye, E.B.; Lokman, I.H.; Hezmee, M.N.M.; Goh, Y.M.; Zuki, A.B.Z.; Jimoh, A.A. Extraction and Physicochemical Characterization of Chitin and Chitosan Isolated from House Cricket. Biomed. Mater. 2018, 13, 025009. [Google Scholar] [CrossRef] [Green Version]
- Soon, C.Y.; Tee, Y.B.; Tan, C.H.; Rosnita, A.T.; Khalina, A. Extraction and Physicochemical Characterization of Chitin and Chitosan from Zophobas Morio Larvae in Varying Sodium Hydroxide Concentration. Int. J. Biol. Macromol. 2018, 108, 135–142. [Google Scholar] [CrossRef]
- Moolphuerk, N.; Lawson, T.; Pattanagul, W. Chitosan Mitigates the Adverse Effects and Improves Photosynthetic Activity in Rice (Oryza sativa L.) Seedlings under Drought Condition. J. Crop Improv. 2022, 36, 638–655. [Google Scholar] [CrossRef]
- Minh, N.C.; Van Hoa, N.; Trung, T.S. Preparation, Properties, and Application of Low-Molecular-Weight Chitosan. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. 453–471. ISBN 978-0-12-817970-3. [Google Scholar]
- Chang, S.-H.; Wu, C.-H.; Tsai, G.-J. Effects of Chitosan Molecular Weight on Its Antioxidant and Antimutagenic Properties. Carbohydr. Polym. 2018, 181, 1026–1032. [Google Scholar] [CrossRef]
- Trinetta, V.; McDaniel, A.; G Batziakas, K.; Yucel, U.; Nwadike, L.; Pliakoni, E. Antifungal Packaging Film to Maintain Quality and Control Postharvest Diseases in Strawberries. Antibiotics 2020, 9, 618. [Google Scholar] [CrossRef]
- Chaiwong, N.; Leelapornpisid, P.; Jantanasakulwong, K.; Rachtanapun, P.; Seesuriyachan, P.; Sakdatorn, V.; Leksawasdi, N.; Phimolsiripol, Y. Antioxidant and Moisturizing Properties of Carboxymethyl Chitosan with Different Molecular Weights. Polymers 2020, 12, 1445. [Google Scholar] [CrossRef]





| Chitosan Samples | Mineral Acids | Organic Acids | |||
|---|---|---|---|---|---|
| ChHCl | ChH2SO4 | ChCitric | ChAcetic | ChLactic | |
| Ash | 0.05 ± 0.10 b | 0.03 ± 0.02 a | 0.02 ± 0.07 a | 0.19 ± 0.04 c | 0.50 ± 0.17 d |
| Moisture | 7.83 ± 0.20 d | 7.24 ± 0.06 a | 7.59 ± 0.09 c | 7.85 ± 1.00 d | 7.52 ± 1.02 b |
| Chitosan Samples | Mineral Acids | Organic Acids | |||
|---|---|---|---|---|---|
| ChHCl | ChH2SO4 | ChCitric | ChAcetic | ChLactic | |
| WBC (%) | 554 ± 11.3 a | 596 ± 09.8 c | 601 ± 14.6 c | 638 ± 11.0 d | 587 ± 13.8 b |
| FBC (%) | 429 ± 21.0 c | 442 ± 16.5 e | 437 ± 10.8 d | 384 ± 11.4 a | 420 ± 17.2 b |
| Chitosan Samples | Mineral Acids | Organic Acids | |||
|---|---|---|---|---|---|
| ChHCl | ChH2SO4 | ChCitric | ChAcetic | ChLactic | |
| Solubility | 78.45 ± 0.10 d | 70.22 ± 0.03 b | 74.18 ± 0.20 c | 69.02 ± 0.01 b | 60.29 ± 0.02 a |
| Chitosan Samples | Mineral Acids | Organic Acids | |||
|---|---|---|---|---|---|
| ChHCl | ChH2SO4 | ChCitric | ChAcetic | ChLactic | |
| DD FTIR | 83.67 ± 0.6 e | 80.23 ± 0.0 c | 81.47 ± 0.4 d | 77.83 ± 0.3 b | 69.14 ± 1.2 a |
| DD Titration | 85.61 ± 0.2 c | 79.84 ± 0.6 b | 85.20 ± 0.9 c | 78.64 ± 1.0 b | 72.92 ± 0.0 a |
| Chitosan Samples | Mineral Acids | Organic Acids | |||
|---|---|---|---|---|---|
| ChHCl | ChH2SO4 | ChCitric | ChAcetic | ChLactic | |
| [η] (dL/g) | 2.6943 | 1.9179 | 2.2559 | 2.6490 | 2.9336 |
| Mw (kDa) | 229.184 | 149.047 | 183.044 | 224.317 | 255.248 |
| Functional Groups and Vibration Modes | ChHCl | ChH2SO4 | ChCitric | ChAcetic | ChLactic |
|---|---|---|---|---|---|
| Stretching vibrations of hydroxyl groups (OH) Asymmetric/symmetric stretching of the amine bonds (NH2) | 3357.91 | 3328.98 | 3263.40 | 3421.55 | 3358.63 |
| C-H aliphatic stretching vibration (CH2) | 2920.09 2877.65 | 2918.16 2871.87 | 2920.38 2885.37 | 2921.59 2870.21 | 2919.25 2869.94 |
| Amide frequencies of C=O bond stretching of amide I | 1656.77 | 1652.91 | 1649.70 | 1620.63 | 1658.13 |
| N-H bending vibrations of NH2 groups of the amide II | 1568.05 | 1587.34 | 1556.48 | 1558.41 | 1584.41 |
| CH2 deformation vibrations in the CH2OH groups | 1419.54 | 1421.47 | 1431.11 | 1425.32 | 1404.11 |
| Symmetrical angular deformation of CH3 in NHCOCH3 groups | 1375.18 | 1377.11 | 1380.96 | 1380.43 | 1369.39 |
| C-N stretching vibrations of amide III | 1317.31 1259.45 | 1318.24 1256.60 | 1303.81 1256.60 | 1317.31 1258.52 | 1298.03 1256.07 |
| Symmetric/asymmetric stretching signals of the C-O-C bridge (glycosidic linkage) | 1149.52 1060.79 | 1151.44 1064.65 | 1253.67 1062.72 | 1153.37 1066.58 | 1163.02 1062.44 |
| C-O stretching vibration in secondary and primary OH groups | 1026.08 985.57 | 1022.29 941.21 | 1018.36 985.89 | 1021.22 985.77 | 1004.86 968.37 |
| Chitosan Samples | Mineral Acids | Organic Acids | |||
|---|---|---|---|---|---|
| ChHCl | ChH2SO4 | ChCitric | ChAcetic | ChLactic | |
| CrI | 87.43% | 82.61% | 84.51% | 82.02% | 82.24% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-araby, A.; El Ghadraoui, L.; Errachidi, F. Physicochemical Properties and Functional Characteristics of Ecologically Extracted Shrimp Chitosans with Different Organic Acids during Demineralization Step. Molecules 2022, 27, 8285. https://doi.org/10.3390/molecules27238285
El-araby A, El Ghadraoui L, Errachidi F. Physicochemical Properties and Functional Characteristics of Ecologically Extracted Shrimp Chitosans with Different Organic Acids during Demineralization Step. Molecules. 2022; 27(23):8285. https://doi.org/10.3390/molecules27238285
Chicago/Turabian StyleEl-araby, Abir, Lahsen El Ghadraoui, and Faouzi Errachidi. 2022. "Physicochemical Properties and Functional Characteristics of Ecologically Extracted Shrimp Chitosans with Different Organic Acids during Demineralization Step" Molecules 27, no. 23: 8285. https://doi.org/10.3390/molecules27238285
APA StyleEl-araby, A., El Ghadraoui, L., & Errachidi, F. (2022). Physicochemical Properties and Functional Characteristics of Ecologically Extracted Shrimp Chitosans with Different Organic Acids during Demineralization Step. Molecules, 27(23), 8285. https://doi.org/10.3390/molecules27238285