Facile Synthesis of ZIF-67 for the Adsorption of Methyl Green from Wastewater: Integrating Molecular Models and Experimental Evidence to Comprehend the Removal Mechanism
Abstract
:1. Introduction
2. Experimental
2.1. Materials
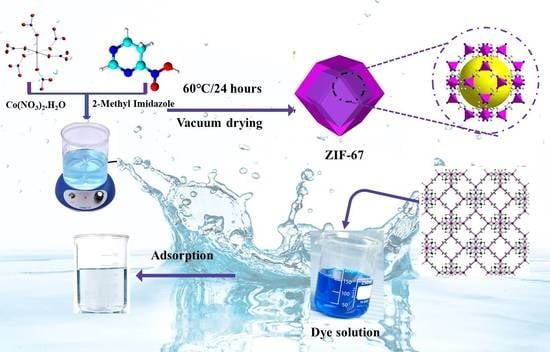
2.2. Synthesis of Materials
2.3. Characterization
2.4. Batch Adsorption Experiment
2.5. Adsorption Kinetics
2.6. Adsorption Isotherm
2.7. Effect of Different Parameters
3. Results
4. Adsorption Experiments
4.1. Effect of pH
4.2. Effect of Contact Time and MG Concentration
4.3. Effect of Initial Adsorbate Concentration
4.4. Adsorption Kinetics
4.5. Adsorption Isotherms
4.6. Reusability of ZIF-67
4.7. Examining the Adsorption Mechanism Using DFT Analysis
4.8. Adsorption Mechanism
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Naseri, A.; Asghari Sarabi, G.; Samadi, M.; Yousefi, M.; Ebrahimi, M.; Moshfegh, A.Z. Recent advances on dual-functional photocatalytic systems for combined removal of hazardous water pollutants and energy generation. Res. Chem. Intermed. 2022, 48, 911–933. [Google Scholar] [CrossRef]
- Hojjati-Najafabadi, A.; Mansoorianfar, M.; Liang, T.; Shahin, K.; Karimi-Maleh, H. A review on magnetic sensors for monitoring of hazardous pollutants in water resources. Sci. Total Environ. 2022, 824, 153844. [Google Scholar] [CrossRef]
- Khan, M.A.; Mutahir, S.; Wang, F.; Lei, W.; Xia, M. Sensitization of TiO2 nanosheets with Cu–biphenylamine framework to enhance photocatalytic degradation performance of toxic organic contaminants: Synthesis, mechanism and kinetic studies. Nanotechnology 2018, 29, 375605. [Google Scholar] [CrossRef] [PubMed]
- Beydaghdari, M.; Saboor, F.H.; Babapoor, A.; Asgari, M. Recent Progress in Adsorptive Removal of Water Pollutants by Metal-Organic Frameworks. ChemNanoMat 2022, 8, e202100400. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Aravindakumar, C.T.; Shah, N.S.; Boczkaj, G. Advanced oxidation processes (AOPs) based wastewater treatment-unexpected nitration side reactions-a serious environmental issue: A review. Chem. Eng. J. 2022, 430, 133002. [Google Scholar] [CrossRef]
- Khader, E.H.; Mohammed, T.J.; Mirghaffari, N.; Salman, A.D.; Juzsakova, T.; Abdullah, T.A. Removal of organic pollutants from produced water by batch adsorption treatment. Clean Technol. Environ. Policy 2022, 24, 713–720. [Google Scholar] [CrossRef]
- Alalwan, H.A.; Mohammed, M.M.; Sultan, A.J.; Abbas, M.N.; Ibrahim, T.A.; Aljaafari, H.A.; Alminshid, A.A. Adsorption of methyl green stain from aqueous solutions using non-conventional adsorbent media: Isothermal kinetic and thermodynamic studies. Bioresour. Technol. Rep. 2021, 14, 100680. [Google Scholar] [CrossRef]
- Iqbal, M.; Bhatti, H.N.; Younis, S.; Rehmat, S.; Alwadai, N.; Almuqrin, A.H.; Iqbal, M. Graphene oxide nanocomposite with CuSe and photocatalytic removal of methyl green dye under visible light irradiation. Diam. Relat. Mater. 2021, 113, 108254. [Google Scholar] [CrossRef]
- Motamedi, E.; Kavousi, K.; Motahar, S.F.S.; Ghaffari, M.R.; Mamaghani, A.S.A.; Salekdeh, G.H.; Ariaeenejad, S. Efficient removal of various textile dyes from wastewater by novel thermo-halotolerant laccase. Bioresour. Technol. 2021, 337, 125468. [Google Scholar] [CrossRef]
- Yang, L.; Zhan, Y.; Gong, Y.; Ren, E.; Lan, J.; Guo, R.; Yan, B.; Chen, S.; Lin, S. Development of eco-friendly CO2-responsive cellulose nanofibril aerogels as “green” adsorbents for anionic dyes removal. J. Hazard. Mater. 2021, 405, 124194. [Google Scholar] [CrossRef]
- Jain, N.; Gupta, E.; Kanu, N.J. Plethora of Carbon nanotubes applications in various fields—A state-of-the-art-review. Smart Sci. 2022, 10, 1–24. [Google Scholar] [CrossRef]
- Li, Z.; Wu, G.; Yang, Y.; Wan, Z.; Zeng, X.; Yan, L.; Wu, S.; Ling, M.; Liang, C.; Hui, K.N. An Ion-Conductive Grafted Polymeric Binder with Practical Loading for Silicon Anode with High Interfacial Stability in Lithium-Ion Batteries. Adv. Energy Mater. 2022, 12, 2201197. [Google Scholar] [CrossRef]
- Habiba, U.; Mutahir, S.; Khan, M.A.; Humayun, M.; Refat, M.S.; Munawar, K.S. Effective Removal of Refractory Pollutants through Cinnamic Acid-Modified Wheat Husk Biochar: Experimental and DFT-Based Analysis. Catalysts 2022, 12, 1063. [Google Scholar] [CrossRef]
- Mutahir, S.; Irfan, T.; Nadeem, N.; Humayun, M.; Khan, M.A.; Refat, M.S.; Wang, C.; Sheikh, T.A. Synthesis and Micromechanistic Studies of Sensitized Bentonite for Methyl Orange and Rhodamine-B Adsorption from Wastewater: Experimental and DFT-Based Analysis. Molecules 2022, 27, 5567. [Google Scholar] [CrossRef] [PubMed]
- Tabassam, N.; Mutahir, S.; Khan, M.A.; Khan, I.U.; Habiba, U.; Refat, M.S. Facile synthesis of cinnamic acid sensitized rice husk biochar for removal of organic dyes from wastewaters: Batch experimental and theoretical studies. Mater. Chem. Phys. 2022, 288, 126327. [Google Scholar] [CrossRef]
- Sun, D.; Wang, D.; Dang, Y.; Zhang, S.; Chen, H.; Hou, R.; Wu, K.; Shen, C. Organic–Inorganic Hybrid Noncentrosymmetric (Morpholinium)2Cd2Cl6 Single Crystals: Synthesis, Nonlinear Optical Properties, and Stability. Inorg. Chem. 2022, 61, 8076–8082. [Google Scholar] [CrossRef]
- Adil, H.I.; Thalji, M.R.; Yasin, S.A.; Saeed, I.A.; Assiri, M.A.; Chong, K.F.; Ali, G.A. Metal–organic frameworks (MOFs) based nanofiber architectures for the removal of heavy metal ions. Rsc Adv. 2022, 12, 1433–1450. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, N.; Yue, Y.; Xiao, J.; Huang, X.; Ishag, A. Recent advances on the application of zeolitic imidazolate frameworks (ZIFs) in environmental remediation: A review. Environ. Sci. Nano 2022, 426, 131733. [Google Scholar] [CrossRef]
- Ahmadi, S.; Kalaee, M.; Moradi, O.; Nosratinia, F.; Abdouss, M. Synthesis of novel zeolitic imidazolate framework (ZIF-67)–zinc oxide (ZnO) nanocomposite (ZnO@ZIF-67) and potential adsorption of pharmaceutical (tetracycline (TCC)) from water. J. Mol. Struct. 2022, 1251, 132013. [Google Scholar] [CrossRef]
- Valadi, F.M.; Ekramipooya, A.; Gholami, M.R. Selective separation of Congo Red from a mixture of anionic and cationic dyes using magnetic-MOF: Experimental and DFT study. J. Mol. Liq. 2020, 318, 114051. [Google Scholar] [CrossRef]
- Nazir, M.A.; Khan, N.A.; Cheng, C.; Shah, S.S.A.; Najam, T.; Arshad, M.; Sharif, A.; Akhtar, S.; ur Rehman, A. Surface induced growth of ZIF-67 at Co-layered double hydroxide: Removal of methylene blue and methyl orange from water. Appl. Clay Sci. 2020, 190, 105564. [Google Scholar] [CrossRef]
- Guediri, A.; Bouguettoucha, A.; Chebli, D.; Chafai, N.; Amrane, A. Molecular dynamic simulation and DFT computational studies on the adsorption performances of methylene blue in aqueous solutions by orange peel-modified phosphoric acid. J. Mol. Struct. 2020, 1202, 127290. [Google Scholar] [CrossRef]
- Han, X.; Chu, L.; Liu, S.; Chen, T.; Ding, C.; Yan, J.; Cui, L.; Quan, G. Removal of methylene blue from aqueous solution using porous biochar obtained by KOH activation of peanut shell biochar. BioResources 2015, 10, 2836–2849. [Google Scholar] [CrossRef] [Green Version]
- Zhen, M.; Tang, J.; Song, B.; Liu, X. Decontamination of methylene blue from aqueous solution by rhamnolipid-modified biochar. BioResources 2018, 13, 3061–3081. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Hu, X.; Yan, X.; Feng, R.; Zhou, M.; Xue, J. Enhanced adsorption of Rhodamine B by magnetic nitrogen-doped porous carbon prepared from bimetallic ZIFs. Colloids Surf. A Physicochem. Eng. Asp. 2019, 575, 10–17. [Google Scholar] [CrossRef]
- Zhong, G.; Liu, D.; Zhang, J. The application of ZIF-67 and its derivatives: Adsorption, separation, electrochemistry and catalysts. J. Mater. Chem. A 2018, 6, 1887–1899. [Google Scholar] [CrossRef]
- Nazir, M.A.; Bashir, M.S.; Jamshaid, M.; Anum, A.; Najam, T.; Shahzad, K.; Imran, M.; Shah, S.S.A.; ur Rehman, A. Synthesis of porous secondary metal-doped MOFs for removal of Rhodamine B from water: Role of secondary metal on efficiency and kinetics. Surf. Interfaces 2021, 25, 101261. [Google Scholar] [CrossRef]
- Khan, I.; Yuan, A.; Khan, S.; Khan, A.; Khan, S.; Shah, S.A.; Luo, M.; Yaseen, W.; Shen, X.; Yaseen, M. Graphitic Carbon Nitride Composites with Gold and ZIF-67 Nanoparticles as Visible-Light-Promoted Catalysts for CO2 Conversion and Bisphenol A Degradation. ACS Appl. Nano Mater. 2022, 5, 13404–13416. [Google Scholar] [CrossRef]
- Bashandeh, Z.; Hachem, K.; Khalaji, A.D.; Alsaikhan, F.; Bokov, D.O. Removal of methyl green using new modified epichlorohydrine chitosan Schiff base as an efficient adsorbent. Cellulose 2022, 29, 5177–5189. [Google Scholar] [CrossRef]
- Li, Y.; Yan, X.; Hu, X.; Feng, R.; Zhou, M. Trace pyrolyzed ZIF-67 loaded activated carbon pellets for enhanced adsorption and catalytic degradation of Rhodamine B in water. Chem. Eng. J. 2019, 375, 122003. [Google Scholar] [CrossRef]
- Dos Reis, L.G.T.; Robaina, N.F.; Pacheco, W.F.; Cassella, R.J. Separation of Malachite Green and Methyl Green cationic dyes from aqueous medium by adsorption on Amberlite XAD-2 and XAD-4 resins using sodium dodecylsulfate as carrier. Chem. Eng. J. 2011, 171, 532–540. [Google Scholar] [CrossRef]
- Satlaoui, Y.; Trifi, M.; Fkih Romdhane, D.; Charef, A.; Azouzi, R. Removal Properties, Mechanisms, and Performance of Methyl Green from Aqueous Solution Using Raw and Purified Sejnane Clay Type. J. Chem. 2019, 2019, 4121864. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Wang, X.; Zhao, L.; Li, M.; Yang, W. Effective removal of dyes from aqueous solutions by a gelatin hydrogel. J. Polym. Environ. 2021, 29, 3497–3508. [Google Scholar] [CrossRef]
- Yu, K.L.; Lee, X.J.; Ong, H.C.; Chen, W.-H.; Chang, J.-S.; Lin, C.-S.; Show, P.L.; Ling, T.C. Adsorptive removal of cationic methylene blue and anionic Congo red dyes using wet-torrefied microalgal biochar: Equilibrium, kinetic and mechanism modeling. Environ. Pollut. 2021, 272, 115986. [Google Scholar] [CrossRef] [PubMed]
- Korde, S.; Deshmukh, S.; Tandekar, S.; Jugade, R. Implementation of response surface methodology in physi-chemisorption of Indigo carmine dye using modified chitosan composite. Carbohydr. Polym. Technol. Appl. 2021, 2, 100081. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; El-Sayed, T.; Alnoman, R. Mesophase behavior and DFT conformational analysis of new symmetrical diester chalcone liquid crystals. J. Mol. Liq. 2019, 285, 96–105. [Google Scholar] [CrossRef]
- Ferkous, H.; Rouibah, K.; Hammoudi, N.-E.-H.; Alam, M.; Djilani, C.; Delimi, A.; Laraba, O.; Yadav, K.K.; Ahn, H.-J.; Jeon, B.-H. The removal of a textile dye from an aqueous solution using a biocomposite adsorbent. Polymers 2022, 14, 2396. [Google Scholar] [CrossRef]
- Bai, K.-P.; Zhou, L.-J.; Yang, G.-P.; Cao, M.-X.; Wang, Y.-Y. Four new metal-organic frameworks based on diverse metal clusters: Syntheses, structures, luminescent sensing and dye adsorption properties. J. Solid State Chem. 2020, 287, 121336. [Google Scholar] [CrossRef]
- Alardhi, S.M.; Albayati, T.M.; Alrubaye, J.M. Adsorption of the methyl green dye pollutant from aqueous solution using mesoporous materials MCM-41 in a fixed-bed column. Heliyon 2020, 6, e03253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Yang, G.-P.; Zhang, P.-F.; Ma, L.-L.; Wang, J.-M.; Li, G.-P.; Wang, Y.-Y. Microporous Cd(II) Metal–Organic Framework for CO2 Catalysis, Luminescent Sensing, and Absorption of Methyl Green. Cryst. Growth Des. 2021, 21, 2734–2743. [Google Scholar] [CrossRef]
- Cheng, S.; Wu, Y.; Jin, J.; Liu, J.; Wu, D.; Yang, G.; Wang, Y.-Y. New multifunctional 3D porous metal–organic framework with selective gas adsorption, efficient chemical fixation of CO2 and dye adsorption. Dalton Trans. 2019, 48, 7612–7618. [Google Scholar] [CrossRef] [PubMed]







| Parameters (eV) | ZIF-67 | MG |
|---|---|---|
| ELUMO | −2.76 | −7.14 |
| EHOMO | −5.35 | −0.019 |
| Energy gap EHOMO − ELUMO | 2.59 | 7.121 |
| Ionization potential (I = −EHOMO) | 2.76 | 7.14 |
| Electron Affinity (A = −ELUMO) | 5.35 | 0.019 |
| Chemical Hardness (η = (I − A)/2) | −1.295 | 3.5605 |
| Chemical Softness (ζ = 1/2η) | 0.6475 | 1.78025 |
| Electronegativity (χ = (I + A)/2) | 4.055 | 7.159 |
| Chemical Potential (µ = −(I + A)/2) | −4.055 | −7.159 |
| Electrophilicity Index (ω = µ2/2η) | −6.3486 | −7.972 |
| Sr. No. | Adsorbent | The Maximum Adsorption Capacity qmax (mg/g) | References |
|---|---|---|---|
| 1 | MOF with [Zn4O] Clusters | 3.45 | [40] |
| 2 | MCM-41 | 20.97 | [41] |
| 3 | MOF, {[Cd(Hbptc)]·H2O}n (Cdbptc) | 63.5 | [42] |
| 4 | MOF, [Mn4(L)2(H2O)4]n·4DMF·H2O | 25.81 | [43] |
| 5 | ZIF-76 | 96.5 | Present Work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikram, M.; Mutahir, S.; Humayun, M.; Khan, M.A.; Al-Humaidi, J.Y.; Refat, M.S.; Abouzied, A.S. Facile Synthesis of ZIF-67 for the Adsorption of Methyl Green from Wastewater: Integrating Molecular Models and Experimental Evidence to Comprehend the Removal Mechanism. Molecules 2022, 27, 8385. https://doi.org/10.3390/molecules27238385
Ikram M, Mutahir S, Humayun M, Khan MA, Al-Humaidi JY, Refat MS, Abouzied AS. Facile Synthesis of ZIF-67 for the Adsorption of Methyl Green from Wastewater: Integrating Molecular Models and Experimental Evidence to Comprehend the Removal Mechanism. Molecules. 2022; 27(23):8385. https://doi.org/10.3390/molecules27238385
Chicago/Turabian StyleIkram, Muniba, Sadaf Mutahir, Muhammad Humayun, Muhammad Asim Khan, Jehan Y. Al-Humaidi, Moamen S. Refat, and Amr S. Abouzied. 2022. "Facile Synthesis of ZIF-67 for the Adsorption of Methyl Green from Wastewater: Integrating Molecular Models and Experimental Evidence to Comprehend the Removal Mechanism" Molecules 27, no. 23: 8385. https://doi.org/10.3390/molecules27238385
APA StyleIkram, M., Mutahir, S., Humayun, M., Khan, M. A., Al-Humaidi, J. Y., Refat, M. S., & Abouzied, A. S. (2022). Facile Synthesis of ZIF-67 for the Adsorption of Methyl Green from Wastewater: Integrating Molecular Models and Experimental Evidence to Comprehend the Removal Mechanism. Molecules, 27(23), 8385. https://doi.org/10.3390/molecules27238385