Gallic Acid-Loaded Sodium Alginate-Based (Polyvinyl Alcohol-Co-Acrylic Acid) Hydrogel Membranes for Cutaneous Wound Healing: Synthesis and Characterization
Abstract
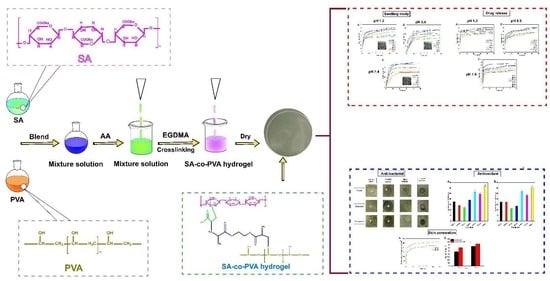
:1. Introduction
2. Results and Discussion
2.1. FTIR
2.2. TGA Study
2.3. DSC Study
2.4. XRD Analysis
2.5. SEM Analysis
2.6. Mechanical Properties Analysis
2.7. Sol–Gel Analysis
2.8. Porosity Study
2.9. Biodegradation Analysis
2.10. Swelling Behavior
2.11. Release and Kinetic Modelling
2.12. Structural Parameters of Hydrogel Membranes
2.13. Antioxidation Analysis
2.14. Antibacterial Study
2.15. Dermal Irritation Study
2.16. In Vitro Permeation and Skin Deposition Study
3. Material and Methods
3.1. Materials
3.2. Preparation of Hydrogel Membrane
Drug Loading into the Hydrogel Membranes
3.3. Characterization
3.3.1. FTIR Analysis
3.3.2. Thermogravimetric Analysis
3.3.3. Differential Scanning Calorimetry
3.3.4. X-ray Diffraction Study
3.3.5. Scanning Electron Microscopy
3.3.6. Evaluation of Mechanical Properties
3.3.7. Sol–Gel Analysis
3.3.8. Porosity Study
3.3.9. Biodegradation Study
3.4. Hydrogel Membrane Network Parameters
3.5. Equilibrium Swelling Ratio (ESR)
3.6. Release and Kinetic Modelling
3.7. Antioxidant Studies
3.7.1. DPPH Antioxidant Activity
3.7.2. ABTS Antioxidant Activity
3.8. Investigation of Antibacterial Activity
3.9. Acute Dermal Irritation Test
3.10. In Vitro Permeation and Skin Deposition Study
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kar, A.; Ahamad, N.; Dewani, M.; Awasthi, L.; Patil, R.; Banerjee, R. Wearable and implantable devices for drug delivery: Applications and challenges. Biomaterials 2022, 283, 121435. [Google Scholar] [CrossRef] [PubMed]
- Abazari, M.; Ghaffari, A.; Rashidzadeh, H.; Badeleh, S.M.; Maleki, Y. A systematic review on classification, identification, and healing process of burn wound healing. Int. J. Low. Extrem. Wounds 2022, 21, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.E.; Foster, D.S.; Longaker, M.T. Management of chronic wounds—2018. JAMA 2018, 320, 1481–1482. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment strategies for infected wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buch, P.J.; Chai, Y.; Goluch, E.D. Treating polymicrobial infections in chronic diabetic wounds. Clin. Microbiol. Rev. 2019, 32, e00091-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Behl, T.; Chadha, S. A rationalized and innovative perspective of nanotechnology and nanobiotechnology in chronic wound management. J. Drug Deliv. Sci. Technol. 2020, 60, 101930. [Google Scholar] [CrossRef]
- Kim, H.S.; Sun, X.; Lee, J.-H.; Kim, H.-W.; Fu, X.; Leong, K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 2019, 146, 209–239. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, X.; Meng, S. Site-specific drug delivery in the skin for the localized treatment of skin diseases. Expert Opin. Drug Deliv. 2019, 16, 847–867. [Google Scholar] [CrossRef]
- Fu, L.Q.; Chen, X.Y.; Cai, M.H.; Tao, X.H.; Fan, Y.B.; Mou, X.Z. Surface Engineered Metal-Organic Frameworks (MOFs) Based Novel Hybrid Systems for Effective Wound Healing: A Review of Recent Developments. Front. Bioeng. Biotechnol. 2020, 8, 576348. [Google Scholar] [CrossRef]
- Zhong, Y.; Xiao, H.; Seidi, F.; Jin, Y. Natural polymer-based antimicrobial hydrogels without synthetic antibiotics as wound dressings. Biomacromolecules 2020, 21, 2983–3006. [Google Scholar] [CrossRef]
- Karimi, M.; Mehrabadi, Z.; Farsadrooh, M.; Bafkary, R.; Derikvandi, H.; Hayati, P.; Mohammadi, K. Chapter 4—Metal–organic framework. In Interface Science and Technology; Ghaedi, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 33, pp. 279–387. [Google Scholar]
- Jafari, S.; Izadi, Z.; Alaei, L.; Jaymand, M.; Samadian, H.; Kashani, V.o.; Derakhshankhah, H.; Hayati, P.; Noori, F.; Mansouri, K.; et al. Human plasma protein corona decreases the toxicity of pillar-layer metal organic framework. Sci. Rep. 2020, 10, 14569. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lu, J.; You, T.; Sun, D. Metal-organic frameworks for improving wound healing. Coord. Chem. Rev. 2021, 439, 213929. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [Green Version]
- Fan, F.; Saha, S.; Hanjaya-Putra, D. Biomimetic hydrogels to promote wound healing. Front. Bioeng. Biotechnol. 2021, 9, 718377. [Google Scholar] [CrossRef] [PubMed]
- Samadian, H.; Zamiri, S.; Ehterami, A.; Farzamfar, S.; Vaez, A.; Khastar, H.; Alam, M.; Ai, A.; Derakhshankhah, H.; Allahyari, Z.; et al. Electrospun cellulose acetate/gelatin nanofibrous wound dressing containing berberine for diabetic foot ulcer healing: In vitro and in vivo studies. Sci. Rep. 2020, 10, 8312. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Saboury, A.A.; Tajerzadeh, H.; Hayati, P.; Dehghanian, M.; Soorbaghi, F.P.; Ghorbani, M.; Kashani, V.O.; Derakhshankhah, H. Optimization and development of drug loading in hydroxyapatite–polyvinyl alcohol nanocomposites via response surface modeling approach. J. Iran. Chem. Soc. 2020, 17, 1141–1151. [Google Scholar] [CrossRef]
- Cui, R.; Zhang, L.; Ou, R.; Xu, Y.; Xu, L.; Zhan, X.-Y.; Li, D. Polysaccharide-based hydrogels for wound dressing: Design considerations and clinical applications. Front. Bioeng. Biotechnol. 2022, 10, 845735. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef] [Green Version]
- Alven, S.; Aderibigbe, B.A. Fabrication of Hybrid Nanofibers from Biopolymers and Poly (Vinyl Alcohol)/Poly (ε-Caprolactone) for Wound Dressing Applications. Polymers 2021, 13, 2104. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging role of hydrogels in drug delivery systems, tissue engineering and wound management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef]
- Gan, J.E.; Chin, C.Y. Formulation and characterisation of alginate hydrocolloid film dressing loaded with gallic acid for potential chronic wound healing. F1000Research 2021, 10, 451. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.K.; Vatanpour, V.; Taghizadeh, A.; Taghizadeh, M.; Ganjali, M.R.; Munir, M.T.; Habibzadeh, S.; Saeb, M.R.; Ghaedi, M. Hydrogel membranes: A review. Mater. Sci. Eng. C 2020, 114, 111023. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ma, X.; Guo, N. Sodium alginate/Na+-rectorite composite microspheres: Preparation, characterization, and dye adsorption. Carbohydr. Polym. 2012, 90, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Koosha, M.; Raoufi, M.; Moravvej, H. One-pot reactive electrospinning of chitosan/PVA hydrogel nanofibers reinforced by halloysite nanotubes with enhanced fibroblast cell attachment for skin tissue regeneration. Colloids Surf. B Biointerfaces 2019, 179, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Al-Sahaf, Z.; Raimi-Abraham, B.; Licciardi, M.; de Mohac, L.M. Influence of Polyvinyl Alcohol (PVA) on PVA-Poly-N-hydroxyethyl-aspartamide (PVA-PHEA) Microcrystalline Solid Dispersion Films. AAPS PharmSciTech 2020, 21, 267. [Google Scholar] [CrossRef]
- Vijay, S.; Sati, O.; Majumdar, D.K. Acrylic acid–methyl methacrylate copolymer for oral prolonged drug release. J. Mater. Sci. Mater. Med. 2010, 21, 2583–2592. [Google Scholar] [CrossRef]
- Bajpai, S.; Jhariya, S. Selective removal of Amikacin from simulated polluted water using molecularly imprinting polymer (MIP). J. Macromol. Sci. Part A 2015, 52, 901–911. [Google Scholar] [CrossRef]
- Akhlaq, M.; Maryam, F.; Elaissari, A.; Ullah, H.; Adeel, M.; Hussain, A.; Ramzan, M.; Ullah, O.; Zeeshan Danish, M.; Iftikhar, S. Pharmacokinetic evaluation of quetiapine fumarate controlled release hybrid hydrogel: A healthier treatment of schizophrenia. Drug Deliv. 2018, 25, 916–927. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Li, Y.; Li, L. Enhanced stability and mechanical strength of sodium alginate composite films. Carbohydr. Polym. 2017, 160, 62–70. [Google Scholar] [CrossRef]
- Aydogdu, A.; Sumnu, G.; Sahin, S. Fabrication of gallic acid loaded Hydroxypropyl methylcellulose nanofibers by electrospinning technique as active packaging material. Carbohydr. Polym. 2019, 208, 241–250. [Google Scholar] [CrossRef]
- Koosha, M.; Mirzadeh, H. Electrospinning, mechanical properties, and cell behavior study of chitosan/PVA nanofibers. J. Biomed. Mater. Res. Part A 2015, 103, 3081–3093. [Google Scholar] [CrossRef] [PubMed]
- Raut, N.S.; Deshmukh, P.R.; Umekar, M.J.; Kotagale, N.R. Zinc cross-linked hydroxamated alginates for pulsed drug release. Int. J. Pharm. Investig. 2013, 3, 194. [Google Scholar] [PubMed] [Green Version]
- Sun, F.; Guo, J.; Liu, Y.; Yu, Y. Preparation, characterizations and properties of sodium alginate grafted acrylonitrile/polyethylene glycol electrospun nanofibers. Int. J. Biol. Macromol. 2019, 137, 420–425. [Google Scholar] [CrossRef]
- Teodoro, G.R.; Gontijo, A.V.L.; Borges, A.C.; Tanaka, M.H.; Lima, G.d.M.G.; Salvador, M.J.; Koga-Ito, C.Y. Gallic acid/hydroxypropyl-β-cyclodextrin complex: Improving solubility for application on in vitro/in vivo Candida albicans biofilms. PLoS ONE 2017, 12, e0181199. [Google Scholar] [CrossRef] [Green Version]
- Neo, Y.P.; Ray, S.; Jin, J.; Gizdavic-Nikolaidis, M.; Nieuwoudt, M.K.; Liu, D.; Quek, S.Y. Encapsulation of food grade antioxidant in natural biopolymer by electrospinning technique: A physicochemical study based on zein–gallic acid system. Food Chem. 2013, 136, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Waresindo, W.X.; Luthfianti, H.R.; Edikresnha, D.; Suciati, T.; Noor, F.A.; Khairurrijal, K. A freeze–thaw PVA hydrogel loaded with guava leaf extract: Physical and antibacterial properties. RSC Adv. 2021, 11, 30156–30171. [Google Scholar] [CrossRef] [PubMed]
- Altaf, F.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Akram, M.A.; Butt, M.S.; Noor, T.; Sher, F. Synthesis and characterization of PVA/starch hydrogel membranes incorporating essential oils aimed to be used in wound dressing applications. J. Polym. Environ. 2021, 29, 156–174. [Google Scholar] [CrossRef]
- Zhai, M.; Xu, Y.; Zhou, B.; Jing, W. Keratin-chitosan/n-ZnO nanocomposite hydrogel for antimicrobial treatment of burn wound healing: Characterization and biomedical application. J. Photochem. Photobiol. B Biol. 2018, 180, 253–258. [Google Scholar] [CrossRef]
- Zhang, X.; Miao, F.; Niu, L.; Wei, Y.; Hu, Y.; Lian, X.; Zhao, L.; Chen, W.; Huang, D. Berberine carried gelatin/sodium alginate hydrogels with antibacterial and EDTA-induced detachment performances. Int. J. Biol. Macromol. 2021, 181, 1039–1046. [Google Scholar] [CrossRef]
- Liu, C.; Tripathi, A.K.; Gao, W.; Tsavalas, J.G. Crosslinking in Semi-Batch Seeded Emulsion Polymerization: Effect of Linear and Non-Linear Monomer Feeding Rate Profiles on Gel Formation. Polymers 2021, 13, 596. [Google Scholar] [CrossRef]
- Tabassum, M.; Pervaiz, F.; Shoukat, H. Fabrication and evaluation of gelatin-PVA-co-poly (2-acrylamido-2-methylpropane sulfonic acid)-based hydrogels for extended-release of sitagliptin and metformin by employing response surface methodology. Chem. Pap. 2022, 76, 4081–4097. [Google Scholar] [CrossRef]
- Torres, M.L.; Oberti, T.G.; Fernández, J.M. HEMA and alginate-based chondrogenic semi-interpenetrated hydrogels: Synthesis and biological characterization. J. Biomater. Sci. Polym. Ed. 2020, 32, 504–523. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.R.; Abu Elella, M.H.; Sabaa, M.W. Synthesis, characterization and applications of N-quaternized chitosan/poly(vinyl alcohol) hydrogels. Int. J. Biol. Macromol. 2015, 80, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Chopra, H.; Kumar, S.; Singh, I. Strategies and Therapies for Wound Healing: A Review. Curr. Drug Targets 2022, 23, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, R. The study of factors affecting the swelling of ultrasound-prepared hydrogel. Polym. Bull. 2019, 76, 1023–1039. [Google Scholar] [CrossRef]
- Alupei, I.C.; Popa, M.; Hamcerencu, M.; Abadie, M. Superabsorbant hydrogels based on xanthan and poly (vinyl alcohol): 1. The study of the swelling properties. Eur. Polym. J. 2002, 38, 2313–2320. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Ranjha, N.M.; Hanif, M. Effect of ethylene glycol dimethacrylate on swelling and on metformin hydrochloride release behavior of chemically crosslinked pH–sensitive acrylic acid–polyvinyl alcohol hydrogel. DARU J. Pharm. Sci. 2015, 23, 41. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, A.R.; Sohail, M.; Minhas, M.U.; Khaliq, T.; Kousar, M.; Khan, S.; Hussain, Z.; Munir, A. Bioinspired sodium alginate based thermosensitive hydrogel membranes for accelerated wound healing. Int. J. Biol. Macromol. 2020, 155, 751–765. [Google Scholar] [CrossRef]
- El-Ghany, A.; Nahed, A.; Mahmoud, Z.M. Synthesis, characterization and swelling behavior of high-performance antimicrobial amphoteric hydrogels from corn starch. Polym. Bull. 2021, 78, 6161–6182. [Google Scholar] [CrossRef]
- Ali, A.; Hussain, M.A.; Haseeb, M.T.; Bukhari, S.N.A.; Tabassum, T.; Farid-ul-Haq, M.; Sheikh, F.A. A pH-responsive, biocompatible, and non-toxic citric acid cross-linked polysaccharide-based hydrogel from Salvia spinosa L. offering zero-order drug release. J. Drug Deliv. Sci. Technol. 2022, 69, 103144. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Haider, S.; Raza, M.A.; Shah, S.A.; Abd Razak, S.I.; Kadir, M.R.A.; Subhan, F.; Haider, A. Smart and pH-sensitive rGO/Arabinoxylan/chitosan composite for wound dressing: In-vitro drug delivery, antibacterial activity, and biological activities. Int. J. Biol. Macromol. 2021, 192, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A.; Pervaiz, E.; Janjua, H.A.; Hussain, Z. In-vitro and in-vivo study of superabsorbent PVA/Starch/g-C3N4/Ag@ TiO2 NPs hydrogel membranes for wound dressing. Eur. Polym. J. 2020, 130, 109650. [Google Scholar] [CrossRef]
- Jalil, A.; Khan, S.; Naeem, F.; Haider, M.S.; Sarwar, S.; Riaz, A.; Ranjha, N.M. The structural, morphological and thermal properties of grafted pH-sensitive interpenetrating highly porous polymeric composites of sodium alginate/acrylic acid copolymers for controlled delivery of diclofenac potassium. Des. Monomers Polym. 2017, 20, 308–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choubey, S.; Goyal, S.; Varughese, L.R.; Kumar, V.; Sharma, A.K.; Beniwal, V. Probing gallic acid for its broad spectrum applications. Mini Rev. Med. Chem. 2018, 18, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ren, D.; Chen, Y.; Jiang, M.; Wang, R.; Wang, Y.-G. Effect of sodium alginate addition to resveratrol on acute gouty arthritis. Cell. Physiol. Biochem. 2015, 36, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Wen, P.; Hu, T.-G.; Wen, Y.; Li, K.-E.; Qiu, W.-P.; He, Z.-L.; Wang, H.; Wu, H. Development of Nervilia fordii extract-loaded electrospun pva/pvp nanocomposite for antioxidant packaging. Foods 2021, 10, 1728. [Google Scholar] [CrossRef]
- Yayan, J.; Ghebremedhin, B.; Rasche, K. Cefepime shows good efficacy and no antibiotic resistance in pneumonia caused by Serratia marcescens and Proteus mirabilis–an observational study. BMC Pharmacol. Toxicol. 2016, 17, 10. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; He, J.; Huang, Y. Role of alginate in antibacterial finishing of textiles. Int. J. Biol. Macromol. 2017, 94, 466–473. [Google Scholar] [CrossRef]
- Cerchiara, T.; Luppi, B.; Bigucci, F.; Orienti, I.; Zecchi, V. Physically cross-linked chitosan hydrogels as topical vehicles for hydrophilic drugs. J. Pharm. Pharmacol. 2002, 54, 1453–1459. [Google Scholar] [CrossRef]
- Dorrani, M.; Kaul, M.; Parhi, A.; LaVoie, E.J.; Pilch, D.S.; Michniak-Kohn, B. TXA497 as a topical antibacterial agent: Comparative antistaphylococcal, skin deposition, and skin permeation studies with mupirocin. Int. J. Pharm. 2014, 476, 199–204. [Google Scholar] [CrossRef]
- Ahmad, S.; Minhas, M.U.; Ahmad, M.; Sohail, M.; Khalid, Q.; Abdullah, O. Synthesis and evaluation of topical hydrogel membranes; a novel approach to treat skin disorders. J. Mater. Sci. Mater. Med. 2018, 29, 191. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Minhas, M.U.; Ahmad, M.; Sohail, M.; Abdullah, O.; Badshah, S.F. Preparation and evaluation of skin wound healing chitosan-based hydrogel membranes. AAPS PharmSciTech 2018, 19, 3199–3209. [Google Scholar] [CrossRef] [PubMed]
- Montaser, A.; Rehan, M.; El-Naggar, M.E. pH-Thermosensitive hydrogel based on polyvinyl alcohol/sodium alginate/N-isopropyl acrylamide composite for treating re-infected wounds. Int. J. Biol. Macromol. 2019, 124, 1016–1024. [Google Scholar] [CrossRef]
- Meng, J.; Xie, Y.; Gu, Y.-H.; Yan, X.; Chen, Y.; Guo, X.-J.; Lang, W.-Z. PVDF-CaAlg nanofiltration membranes with dual thin-film-composite (TFC) structure and high permeation flux for dye removal. Sep. Purif. Technol. 2021, 255, 117739. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, C.; Kim, Y.; Jung, S. Dual crosslinked carboxymethyl cellulose/polyacrylamide interpenetrating hydrogels with highly enhanced mechanical strength and superabsorbent properties. Eur. Polym. J. 2020, 127, 109586. [Google Scholar] [CrossRef]
- Nagakawa, Y.; Kato, M.; Suye, S.-i.; Fujita, S. Fabrication of tough, anisotropic, chemical-crosslinker-free poly (vinyl alcohol) nanofibrous cryogels via electrospinning. RSC Adv. 2020, 10, 38045–38054. [Google Scholar] [CrossRef]
- Hu, Y.; Kim, Y.; Hong, I.; Kim, M.; Jung, S. Fabrication of flexible pH-responsive agarose/succinoglycan hydrogels for controlled drug release. Polymers 2021, 13, 2049. [Google Scholar] [CrossRef]
- Sakthiguru, N.; Sithique, M.A. Fabrication of bioinspired chitosan/gelatin/allantoin biocomposite film for wound dressing application. Int. J. Biol. Macromol. 2020, 152, 873–883. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, X.; Huang, H.; Liu, A.; Liu, H.; Abid, N.; Ming, L. Chitosan/zein films incorporated with essential oil nanoparticles and nanoemulsions: Similarities and differences. Int. J. Biol. Macromol. 2022, 208, 983–994. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; He, X.; Fang, A.; Jiang, R.; Wu, T.; Chen, H.; Cao, X.; Liang, P.; Xia, D. A sterile self-assembled sericin hydrogel via a simple two-step process. Polym. Test. 2019, 80, 106016. [Google Scholar] [CrossRef]
- Zou, M.; Jin, R.; Hu, Y.; Zhang, Y.; Wang, H.; Liu, G.; Nie, Y.; Wang, Y. A thermo-sensitive, injectable and biodegradable in situ hydrogel as a potential formulation for uveitis treatment. J. Mater. Chem. B 2019, 7, 4402–4412. [Google Scholar] [CrossRef]
- Richbourg, N.R.; Peppas, N.A. The swollen polymer network hypothesis: Quantitative models of hydrogel swelling, stiffness, and solute transport. Prog. Polym. Sci. 2020, 105, 101243. [Google Scholar] [CrossRef]
- Lv, Q.; Wu, M.; Shen, Y. Enhanced swelling ratio and water retention capacity for novel super-absorbent hydrogel. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123972. [Google Scholar] [CrossRef]
- Khamrai, M.; Banerjee, S.L.; Paul, S.; Samanta, S.; Kundu, P.P. Curcumin entrapped gelatin/ionically modified bacterial cellulose based self-healable hydrogel film: An eco-friendly sustainable synthesis method of wound healing patch. Int. J. Biol. Macromol. 2019, 122, 940–953. [Google Scholar] [CrossRef]
- Yin, X.; Wen, Y.; Li, Y.; Liu, P.; Li, Z.; Shi, Y.; Lan, J.; Guo, R.; Tan, L. Facile fabrication of sandwich structural membrane with a hydrogel nanofibrous mat as inner layer for wound dressing application. Front. Chem. 2018, 6, 490. [Google Scholar] [CrossRef]
- Xu, S.; Shen, Y.; Li, Y. Antioxidant activities of sorghum kafirin alcalase hydrolysates and membrane/gel filtrated fractions. Antioxidants 2019, 8, 131. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.-C.; Lin, D.-J.; Wu, Y.-R.; Chang, C.-W.; Chen, C.-H.; Ko, C.-L.; Chen, W.-C. Characterization of genipin-crosslinked gelatin/hyaluronic acid-based hydrogel membranes and loaded with hinokitiol: In vitro evaluation of antibacterial activity and biocompatibility. Mater. Sci. Eng. C 2019, 105, 110074. [Google Scholar] [CrossRef]
- Mohamad, N.; Amin, M.C.I.M.; Pandey, M.; Ahmad, N.; Rajab, N.F. Bacterial cellulose/acrylic acid hydrogel synthesized via electron beam irradiation: Accelerated burn wound healing in an animal model. Carbohydr. Polym. 2014, 114, 312–320. [Google Scholar] [CrossRef]
- Vanti, G.; Wang, M.; Bergonzi, M.C.; Zhidong, L.; Bilia, A.R. Hydroxypropyl methylcellulose hydrogel of berberine chloride-loaded escinosomes: Dermal absorption and biocompatibility. Int. J. Biol. Macromol. 2020, 164, 232–241. [Google Scholar] [CrossRef]











| F. Codes | pH | Zero Order | First Order | Higuchi Model | Korsmeyer–Peppas Model | ||||
|---|---|---|---|---|---|---|---|---|---|
| Ko (h−1) | r2 | K1 (h−1) | r2 | K2 (h−1) | r2 | r2 | n | ||
| SPA-1 | 1.2 | 1.110 | 0.8624 | 0.010 | 0.7153 | 0.678 | 0.8577 | 0.9780 | 0.314 |
| 5.5 | 0.5830 | 0.7336 | 0.007 | 0.7548 | 3.575 | 0.8801 | 0.9939 | 0.133 | |
| 7.4 | 1.250 | 0.8574 | 0.047 | 0.7281 | 11.598 | 0.8019 | 0.9901 | 0.321 | |
| SPA-2 | 1.2 | 0.958 | 0.8555 | 0.044 | 0.7136 | 12.120 | 0.7775 | 0.9790 | 0.346 |
| 5.5 | 0.422 | 0.8328 | 0.005 | 0.8464 | 2.486 | 0.9384 | 0.9514 | 0.401 | |
| 7.4 | 0.972 | 0.9384 | 0.056 | 0.7999 | 11.147 | 0.8003 | 0.9702 | 0.217 | |
| SPA-3 | 1.2 | 1.456 | 0.8614 | 0.065 | 0.8367 | 15.360 | 0.6891 | 0.9950 | 0.161 |
| 5.5 | 0.359 | 0.7955 | 0.004 | 0.8075 | 2.157 | 0.9195 | 0.9481 | 0.340 | |
| 7.4 | 1.643 | 0.8831 | 0.118 | 0.8455 | 14.028 | 0.7269 | 0.9926 | 0.082 | |
| SPA-4 | 1.2 | 0.678 | 0.8992 | 0.061 | 0.7415 | 14.433 | 0.8232 | 0.9780 | 0.281 |
| 5.5 | 0.550 | 0.8370 | 0.006 | 0.8553 | 3.232 | 0.9393 | 0.9505 | 0.409 | |
| 7.4 | 0.755 | 0.8674 | 0.096 | 0.7868 | 13.002 | 0.8233 | 0.9832 | 0.154 | |
| SPA-5 | 1.2 | 0.809 | 0.8875 | 0.061 | 0.6595 | 13.145 | 0.8126 | 0.9813 | 0.476 |
| 5.5 | 0.501 | 0.8301 | 0.006 | 0.8469 | 2.952 | 0.9359 | 0.9485 | 0.403 | |
| 7.4 | 0.950 | 0.8240 | 0.091 | 0.7226 | 12.386 | 0.8838 | 0.9793 | 0.345 | |
| SPA-6 | 1.2 | 1.004 | 0.7976 | 0.047 | 0.7665 | 12.331 | 0.8219 | 0.9708 | 0.338 |
| 5.5 | 0.481 | 0.7735 | 0.005 | 0.7905 | 2.901 | 2.9034 | 0.9905 | 0.155 | |
| 7.4 | 1.221 | 0.8954 | 0.088 | 0.8004 | 12.143 | 0.8627 | 0.9700 | 0.275 | |
| SPA-7 | 1.2 | 1.167 | 0.7651 | 0.475 | 0.8297 | 17.358 | 0.9181 | 0.9919 | 0.380 |
| 5.5 | 0.436 | 0.7936 | 0.005 | 0.8080 | 2.620 | 0.9177 | 0.9473 | 0.337 | |
| 7.4 | 1.726 | 0.7850 | 0.656 | 0.8896 | 13.529 | 0.9360 | 0.9996 | 0.224 | |
| F. Codes | V2,s | χ | Mc | Mr | N | D × 10−5 (cm2 s−1) |
|---|---|---|---|---|---|---|
| SPA-1 | 0.051 ± 0.013 | 0.517 ± 0.035 | 7322.7 ± 445 | 77.689 ± 16 | 188.513 ± 26 | 0.265 ± 0.025 |
| SPA-2 | 0.078 ± 0.017 | 0.527 ± 0.030 | 3833.3 ± 576 | 76.372 ± 14 | 100.384 ± 30 | 0.252 ± 0.027 |
| SPA-3 | 0.080 ± 0.020 | 0.533 ± 0.038 | 2121.9 ± 654 | 71.700 ± 15 | 59.188 ± 19 | 0.369 ± 0.038 |
| SPA-4 | 0.094 ± 0.014 | 0.533 ± 0.032 | 1605.9 ± 540 | 80.033 ± 17 | 39.982 ± 25 | 0.647 ± 0.061 |
| SPA-5 | 0.072 ± 0.012 | 0.525 ± 0.029 | 7287.7 ± 723 | 82.300 ± 19 | 177.100 ± 57 | 0.251 ± 0.030 |
| SPA-6 | 0.080 ± 0.010 | 0.528 ± 0.034 | 6057.7 ± 589 | 79.433 ± 20 | 152.523 ± 43 | 0.473 ± 0.044 |
| SPA-7 | 0.109 ± 0.022 | 0.539 ± 0.041 | 2258.7 ± 706 | 78.290 ± 10 | 57.700 ± 27 | 0.345 ± 0.032 |
| Formulations | Sodium Alginate (g) | PVA (g) | Acrylic Acid (g) | APS/SHS (g) | EGDMA (g) | Drug Loaded per 1 g of Hydrogel (g) |
|---|---|---|---|---|---|---|
| SPA-1 | 1 | 1.5 | 26 | 0.4/0.4 | 0.5 | 0.617 |
| SPA-2 | 1 | 1.5 | 26 | 0.4/0.4 | 1 | 0.332 |
| SPA-3 | 1 | 1.5 | 26 | 0.4/0.4 | 1.5 | 0.317 |
| SPA-4 | 1.5 | 1.5 | 26 | 0.4/0.4 | 0.5 | 0.514 |
| SPA-5 | 2 | 1.5 | 26 | 0.4/0.4 | 0.5 | 0.501 |
| SPA-6 | 1.5 | 2 | 26 | 0.4/0.4 | 0.5 | 0.488 |
| SPA-7 | 1.5 | 3 | 26 | 0.4/0.4 | 0.5 | 0.414 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naeem, A.; Yu, C.; Zhu, W.; Chen, X.; Wu, X.; Chen, L.; Zang, Z.; Guan, Y. Gallic Acid-Loaded Sodium Alginate-Based (Polyvinyl Alcohol-Co-Acrylic Acid) Hydrogel Membranes for Cutaneous Wound Healing: Synthesis and Characterization. Molecules 2022, 27, 8397. https://doi.org/10.3390/molecules27238397
Naeem A, Yu C, Zhu W, Chen X, Wu X, Chen L, Zang Z, Guan Y. Gallic Acid-Loaded Sodium Alginate-Based (Polyvinyl Alcohol-Co-Acrylic Acid) Hydrogel Membranes for Cutaneous Wound Healing: Synthesis and Characterization. Molecules. 2022; 27(23):8397. https://doi.org/10.3390/molecules27238397
Chicago/Turabian StyleNaeem, Abid, Chengqun Yu, Weifeng Zhu, Xuanbin Chen, Xuan Wu, Lihua Chen, Zhenzhong Zang, and Yongmei Guan. 2022. "Gallic Acid-Loaded Sodium Alginate-Based (Polyvinyl Alcohol-Co-Acrylic Acid) Hydrogel Membranes for Cutaneous Wound Healing: Synthesis and Characterization" Molecules 27, no. 23: 8397. https://doi.org/10.3390/molecules27238397
APA StyleNaeem, A., Yu, C., Zhu, W., Chen, X., Wu, X., Chen, L., Zang, Z., & Guan, Y. (2022). Gallic Acid-Loaded Sodium Alginate-Based (Polyvinyl Alcohol-Co-Acrylic Acid) Hydrogel Membranes for Cutaneous Wound Healing: Synthesis and Characterization. Molecules, 27(23), 8397. https://doi.org/10.3390/molecules27238397