Distribution, Antioxidant Capacity, Bioavailability and Biological Properties of Anthocyanin Pigments in Blood Oranges and Other Citrus Species
Abstract
:1. Introduction
2. Distribution
2.1. Qualitative Aspects
2.2. Quantitative Aspects
3. Antioxidant Activity
4. Bioavailability
4.1. Glucose Transport Receptors as the Main Agents for the In Vivo Absorption of Glycosylated Cyanidins
4.2. Anthocyanins as Inhibitors of Glucose Uptake
4.3. In-Vivo Intervention Studies
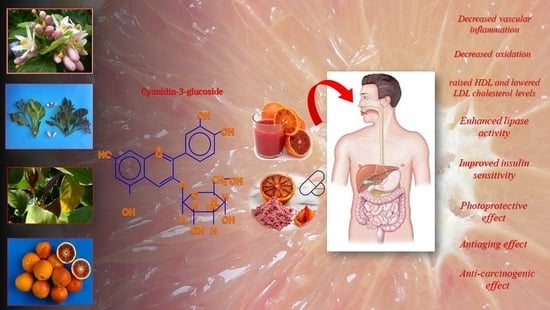
5. Biological Activity
5.1. Metabolic Syndrome, Weight Management, and Obesity
5.2. Heart Health
5.3. Anti-Ageing and Photoprotective Effects
5.4. Anticancer Activity
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hodgson, R.W. History, world distribution, botany and varieties. In The Citrus Industry; Reuther, W., Webber, H.J., Batchelor, L.D., Eds.; University of California: Berkeley, CA, USA, 1967; Volume I, pp. 431–591. [Google Scholar]
- Fabroni, S.; Ballistreri, G.; Amenta, M.; Rapisarda, P. Anthocyanins in different Citrus species: An UHPLC-PDA-ESI/MSn-assisted qualitative and quantitative investigation. J. Sci. Food Agric. 2016, 96, 4797–4808. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Chen, L.L.; Ruan, X.; Chen, D.; Zhu, A.; Chen, C.; Bertran, D.; Jiao, W.B.; Hao, B.H.; Lyon, M.P.; et al. The draft genome of sweet orange (Citrus sinensis). Nat. Genet. 2013, 45, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Gmitter, F.; Hu, X. The possible role of Yunnan province, China, in the origin of contemporary Citrus species (Rutaceae). Econ. Bot. 1990, 44, 267–277. [Google Scholar] [CrossRef]
- Butelli, E.; Licciardello, C.; Zhang, Y.; Liu, J.; Mackay, S.; Bailey, P.; Reforgiato-Recupero, G.; Martin, C. Retrotransposons Control Fruit-Specific, Cold-Dependent Accumulation of Anthocyanins in Blood Oranges. Plant Cell 2012, 24, 1242–1255. [Google Scholar] [CrossRef] [Green Version]
- Yuan, F.; Long, G.; Deng, Z. Jingxian blood orange: The only pigmented sweet orange cultivar originated in China. Abstr. Proc. Int. Soc. Citric. 2008, 1, 70–72. [Google Scholar]
- Rapisarda, P.; Giuffrida, A. Anthocyanins level in Italian blood oranges. Proc. Int. Soc. Citric. 1992, 3, 1130–1133. [Google Scholar]
- Rapisarda, P.; Bellomo, S.E.; Intrigliolo, F. Anthocyanins in blood oranges: Composition and biological activity. Rec. Res. Devel. Agric. Food Chem. 2001, 5, 217–230. [Google Scholar]
- Caruso, M.; Ferlito, F.; Licciardello, C.; Allegra, M.; Strano, M.C.; Di Silvestro, S.; Russo, M.P.; Pietro Paolo, D.; Caruso, P.; Las Casas, G.; et al. Pomological diversity of the Italian blood orange germplasm. Sci. Hortic. 2016, 213, 331–339. [Google Scholar] [CrossRef]
- Starrantino, A.; Reforgiato Recupero, G. Citrus hybrids obtained in vitro from 2x females x 4x males. Proc. Int. Soc. Citric. 1981, 1, 31–32. [Google Scholar]
- Rapisarda, P.; Pannuzzo, P.; Romano, G.; Russo, G. Juice Components of a New pigmented citrus hybrid Citrus sinensis (L.) Osbeck ’x Citrus clementina Hort. ex Tan. J. Agric. Food Chem. 2000, 51, 1611–1616. [Google Scholar] [CrossRef]
- Rapisarda, P.; Bellomo, S.E.; Fabroni, S.; Russo, G. Juice quality of two new mandarin-like hybrids (Citrus clementina Hort. ex Tan x Citrus sinensis L. Osbeck) containing anthocyanins. J. Agric. Food Chem. 2008, 56, 2074–2078. [Google Scholar] [CrossRef] [PubMed]
- Landi, M.; Tattini, M.; Gould, K.S. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Serra, P.; Casati, P. Recent advances on the roles of flavonoids as plant protective molecules after UV and high light exposure. Physiol. Plant. 2021, 173, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Pour, P.M.; Fakhri, S.; Asgary, S.; Farzaei, M.H.; Echeverría, J. The Signaling pathways, and therapeutic targets of antiviral Agents: Focusing on the antiviral approaches and clinical perspectives of anthocyanins in the management of viral diseases. Front. Pharmacol. 2019, 10, 1207. [Google Scholar] [CrossRef]
- Cisowska, A.; Wojnicz, D.; Hendrich, A.B. Anthocyanins as antimicrobial agents of natural plant origin. Nat. Prod. Commun. 2011, 6, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, H.M.; Rentzsch, M.; Breuer, M. Anthocyanins reduce fungal growth in fruits. Nat. Prod. Commun. 2008, 3, 1267–1272. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Fan, L.; He, J.; Wang, Z.; Yin, Y.; Cheng, Y.; Li, Z. Anthocyanins contribute to fruit defense against postharvest green mold. Postharvest Biol. Technol. 2021, 181, 111661. [Google Scholar] [CrossRef]
- Sicilia, A.; Catara, V.; Scialò, E.; Lo Piero, A.R. Fungal Infection Induces Anthocyanin Biosynthesis and Changes in DNA Methylation Configuration of Blood Orange [Citrus sinensis L. (Osbeck)]. Plants 2021, 10, 244. [Google Scholar] [CrossRef]
- Bendokas, V.; Stanys, V.; Mažeikien, I.; Trumbeckaite, S.; Baniene, R.; Liobikas, J. Anthocyanins: From the field to the antioxidants in the body. Antioxidants 2020, 9, 819. [Google Scholar] [CrossRef]
- Rapisarda, P.; Tomaino, A.; Lo Cascio, R.; Bonina, F.; De Pasquale, A.; Saija, A. Antioxidant effectiveness as influence by phenolic content of fresh orange juices. J. Agric. Food Chem. 1999, 47, 4718–4723. [Google Scholar] [CrossRef]
- Grosso, G.; Galvano, F.; Mistretta, A.; Marventano, S.; Nolfo, F.; Calabrese, G.; Buscemi, S.; Drago, F.; Veronesi, U.; Scuderi, A. Red orange: Experimental models and epidemiological evidence of its benefits on human health. Oxidative Med. Cell. Longev. 2013, 2013, 157240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hrazdina, G. Anthocyanins. In The Flavonoids. Advances in Research; Harborne, J.B., Mabry, T.J., Eds.; Chapman and Hall: London, UK; New York, NY, USA, 1982. [Google Scholar]
- Chen, Z.; Deng, H.; Xiong, B.; Li, S.; Yang, L.; Youting, Y.; Huang, S.; Tan, L.; Sun, G.; Wang, Z. Rootstock Effects on Anthocyanin Accumulation and Associated Biosynthetic Gene Expression and Enzyme Activity during Fruit Development and Ripening of Blood Oranges. Crit. Rev. Food Sci. Nutr. 2022, 12, 342. [Google Scholar]
- Castaneda-Ovando, A.; Pacheco-Hernandez, M.; Paez-Hernandez, M.; Rodriguez, J.A.; Galan-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Pourcel, L.; Irani, N.G.; Lu, Y.; Riedl, K.; Steve Schwartzb, S.; Grotewold, E. The formation of anthocyanic vacuolar inclusions in arabidopsis thaliana and implications for the sequestration of anthocyanin pigments. Mol. Plant. 2010, 3, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Habibi, F.; García-Pastor, M.E.; Puente-Moreno, J.; Garrido- Fernando, A.; Serrano, M.; Valero, D. Anthocyanin in blood oranges: A review on postharvest approaches for its enhancement and preservation. Crit. Rev. Food Sci. Nutr. 2022, 1–13. [Google Scholar] [CrossRef]
- Wheldale, M. The Anthocyanin Pigments of Plants; Footnote 1; Cambridge University Press: Cambridge, UK, 1916; p. 27. [Google Scholar]
- Matlack, W. Observation on the red color of the blood orange. Plant Physiol. 1931, 6, 729–730. [Google Scholar] [CrossRef] [Green Version]
- Carrante, V. Sulle pigmentazioni delle arance italiane. Ann. R. Staz. Sper. Frut. Agrum. Acireale 1941, XVI, 193–233. [Google Scholar]
- Ajon, G. The pigments of citrus juice during ripening. Riv. Ital. EPPOS 1943, 25, 150–157. [Google Scholar]
- Patanè, G. Sulla sostanza colorante delle arance sanguigne. Ann. R. Staz. Sper. Frut. Agrum. Acireale 1948, XVII, 1–11. [Google Scholar]
- Chandler, B.V. Anthocyanins of blood orange. Nature 1958, 182, 933. [Google Scholar] [CrossRef]
- Kock, J.; Sajak. Über Naturfarbstoffe in Citrusfrüchten—Anthocyane in blutorangen. Z. Lebensm. Unters Forsch. 1965, 127, 1–4. [Google Scholar] [CrossRef]
- Porretta, A.; Casoli, U.; Dall’Aglio, G. Ricerche sugli antociani del succo d’arancia. Ind. Conserve 1966, 41, 175–179. [Google Scholar]
- Kunkar, A. Le antocianine del succo d’arancio Sanguinello calabrese. Riv. Ital. EPPOS 1968, 50, 180–183. [Google Scholar]
- Licastro, F.; Bellomo, A. Identificazione degli antociani nelle arance Moro, Rass. Chim 1973, 25, 306–310. [Google Scholar]
- Maccarone, E.; Maccarrone, A.; Perrini, G.; Rapisarda, P. Anthocyanins of the Moro orange juice. Ann. Chim. 1983, 73, 533–539. [Google Scholar]
- Maccarone, E.; Maccarrone, A.; Rapisarda, P. Acylated anthocyanins from oranges. Ann. Chim. 1985, 75, 79–86. [Google Scholar]
- Legua, P.; Modica, G.; Porras, I.; Conesa, A.; Continella, A. Bioactive compounds, antioxidant activity and fruit quality evaluation of eleven blood orange cultivars. J. Sci. Food Agric. 2021, 102, 2960–2971. [Google Scholar] [CrossRef]
- Maccarone, E.; Rapisarda, P.; Fanella, F.; Arena, E.; Mondello, L. Cyanidin-3-(6″-malonyl)-β-glucoside. One of the major anthocyanins in blood orange juice. Ital. J. Food Sci. 1998, 10, 367–372. [Google Scholar]
- Dugo, P.; Mondello, L.; Morabito, D.; Dugo, G. Characterization of the anthocyanin fraction of Sicilian blood orange juice by Micro-HPLC-ESI/MS. J. Agric. Food Chem. 2003, 51, 1173–1176. [Google Scholar] [CrossRef]
- Hillebrand, S.; Schwarz, M.; Winterhalter, P. Characterization of anthocyanins and pyranoanthocyanins from blood orange [Citrus sinensis (L.) Osbeck] juice. J. Agric. Food. Chem. 2004, 52, 7331–7338. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Alquézar, B.; Alos, E.; Lado, J.; Zacarias, L. Biochemical bases and molecular regulation of pigmentation in the peel of Citrus fruit. Sci. Hortic. 2013, 163, 46–62. [Google Scholar] [CrossRef]
- Francis, F.J. Analysis of anthocyanins. In Anthocyanins as Food Colors; Markakis, P., Ed.; Academic Press: New York, NY, USA; London, UK, 1982; pp. 181–207. [Google Scholar]
- Fuleki, T.; Francis, F.J. Quantitative methods for anthocyanins. 1. Extraction and determination of total anthocyanin in cranberry. J. Food Sci. 1968, 33, 72–77. [Google Scholar] [CrossRef]
- Fuleki, T.; Francis, F.J. Quantitative methods for anthocyanins. 2. Determination of total anthocyanins and degradation index for cranberry juice. J. Food Sci. 1968, 33, 78–83. [Google Scholar] [CrossRef]
- Rapisarda, P.; Fanella, F.; Maccarone, E. Reliability of analytical methods for determining anthocyanins in blood orange juices. J. Agric. Food Chem. 2000, 48, 2249–2252. [Google Scholar] [CrossRef] [PubMed]
- Rapisarda, P.; Fallico, B.; Izzo, R.; Maccarone, E. A simple and reliable method for determining anthocyanins in blood orange juices. Agrochimica 1994, 38, 157–164. [Google Scholar]
- Rapisarda, P.; Russo, G. Fruit Quality of five ‘Tarocco’ selections grown in Italy. Proc. Int. Soc. Citric. 2000, 1149–1153. [Google Scholar]
- Maccarone, E.; Ferrigno, V.; Longo, M.L.; Rapisarda, P. Effects of light on anthocyanins. Kinetics and photodegradation products in acidic aqueous solutions. Ann. Chim. 1987, 77, 499–508. [Google Scholar]
- Maccarone, E.; Rapisarda, P. Research developments on blood orange juices. Proc. Int. Soc. Citric. 2008, 1, 209. [Google Scholar]
- Rapisarda, P.; Bellomo, S.E.; Intelisano, S. Storage temperature effects on blood orange fruit quality. J. Agric. Food Chem. 2001, 49, 3230–3235. [Google Scholar] [CrossRef]
- Lo Piero, R.; Puglisi, I.; Rapisarda, P.; Petrone, G. Anthocyanins accumulation and related gene expression in red orange fruit induced by low temperature storage. J. Agric. Food Chem. 2005, 53, 9083–9088. [Google Scholar] [CrossRef]
- Rapisarda, P.; Bianco, M.L.; Pannuzzo, P.; Timpanaro, N. Effect of cold storage on vitamin C, phenolics and antioxidant activity of five orange genotypes [Citrus sinensis (L.) Osbeck]. Postharvest Biol. Technol. 2008, 49, 348–354. [Google Scholar] [CrossRef]
- Modica, G.; Pannitteri, C.; Di Guardo, M.; La Malfa, S.; Gentile, A.; Ruberto, G.; Pulvirenti, L.; Parafati, L.; Continella, A.; Siracusa, L. Influence of rootstock genotype on individual metabolic responses and antioxidant potential of blood orange cv. Tarocco Scire. J. Food Compos. Anal. 2022, 105, 104246. [Google Scholar] [CrossRef]
- Carmona, L.; Sulli, M.; Diretto, G.; Alquézar, B.; Alves, M.; Peña, L. Improvement of Antioxidant Properties in Fruit from Two Blood and Blond Orange Cultivars by Postharvest Storage at Low Temperature. Antioxidants 2022, 11, 547. [Google Scholar] [CrossRef] [PubMed]
- Habibi, F.; Guillén, F.; Serrano, M.; Valero, D. Physicochemical Changes, Peel Colour, and Juice Attributes of Blood Orange Cultivars Stored at Different Temperatures. Horticulturae 2021, 7, 320. [Google Scholar] [CrossRef]
- Magalhães, M.L.; De Oliveira Lima, L.C.; da Silva Lunguinho, A.; De Carvalho Selvati Rezende, D.A.; Rodrigues Fernandes Ferreira, V.; Brandão, R.M.; De Souza, J.A.; De Souza, E.C.; De Almeida, K.J.; Nelson, D.L.; et al. Influence of cold storage on the bioactivity properties and the quality of the juice of Moro blood orange (Citrus sinensis (L.) Osbeck). Am. J. Plant Sci. 2019, 10, 24–37. [Google Scholar] [CrossRef] [Green Version]
- Carmona, L.; Alquezar, B.; Diretto, G.; Sevi, F.; Malara, T.; Lafuente, M.T.; Leandro Peňa, L. Curing and low temperature combined post-harvest storage enhances anthocyanin biosynthesis in blood oranges. Food Chem. 2021, 342, 128334. [Google Scholar] [CrossRef]
- Kaur, C.; Kapoor, H.C. Antioxidants in fruits and vegetables—The millennium’s health. Int. J. Food Sci. 2001, 36, 703–725. [Google Scholar] [CrossRef]
- Abeysinghe, D.C.; Li, X.; Sun, C.; Zhang, W.; Zhou, C.; Chen, K. Bioactive compounds and antioxidant capacities in different edible tissues of citrus fruit of four species. Food Chem. 2007, 104, 1338–1344. [Google Scholar] [CrossRef]
- Ghasemi, K.; Ghasemi, Y.; Ebrahimzadeh, M.A. Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pak. J. Pharm. Sci. 2009, 22, 277–281. [Google Scholar]
- Franke, S.I.R.; Ckless, K.; Silveira, J.D.; Rubensam, G.; Brendel, M.; Erdtmann, B. Study of antioxidant and mutagenic activity of different orange juices. Food Chem. 2004, 88, 45–55. [Google Scholar] [CrossRef]
- Gironés-Vilaplana, A.; Moreno, D.A.; García-Viguera, C. Phytochemistry and biological activity of Spanish Citrus fruits. Food Funct. 2014, 5, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Sicari, V.; Pellicanò, T.M.; Giuffrè, A.M.; Zappia, C.; Capocasale, M. Bioactive compounds and antioxidant activity of citrus juices produced from varieties cultivated in Calabria. J. Food Meas. Charact. 2016, 10, 773–780. [Google Scholar] [CrossRef]
- Yoo, K.; Hwang, I.; Park, J.H.; Moon, B. Major phytochemical composition of 3 native Korean citrus varieties and bioactive activity on V79-4 cells induced by oxidative stress. J. Food Sci. 2009, 74, C462–C468. [Google Scholar] [CrossRef] [PubMed]
- De Ancos, B.; Cilla, A.; Barberá, R.; Sánchez-Moreno, C.; Cano, M.P. Influence of orange cultivar and mandarin postharvest storage on polyphenols, ascorbic acid and antioxidant activity during gastrointestinal digestion. Food Chem. 2017, 84, 114–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Ancos, B.; Rodrigo, M.J.; Sánchez-Moreno, C.; Cano, M.P.; Zacarias, L. Effect of high-pressure processing applied as pretreatment on carotenoids, flavonoids and vitamin C in juice of the sweet oranges ‘Navel’ and the red-fleshed ‘Cara Cara’. Food Res. Int. 2020, 132, 109105. [Google Scholar] [CrossRef]
- Gardner, P.T.; White, T.A.C.; McPhail, D.B.; Duthie, G.G. The relative contributions of vitamin C, carotenoids and phenolics to the antioxidant potential of fruit juices. Food Chem. 2000, 68, 471–474. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A. The relative contribution of ascorbic acid and phenolic antioxidants to the total antioxidant activity of orange and apple fruit juices and blackcurrant drink. Food Chem. 1997, 60, 331–337. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the art of anthocyanins: Antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, pharmacology and health benefits of anthocyanins: Anthocyanins and human health. Phytother. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef] [PubMed]
- Rapisarda, P.; Fabroni, S.; Peterek, S.; Russo, G.; Mock, H.P. Juice of new citrus hybrids (Citrus clementina Hort. ex Tan. x C. sinensis L. Osbeck). Food Chem. 2009, 117, 212–218. [Google Scholar] [CrossRef]
- Kelebek, H.; Canbas, A.; Selli, S. Determination of phenolic composition and antioxidant capacity of blood orange juices obtained from cvs. Moro and Sanguinello (Citrus sinensis (L.) Osbeck) grown in Turkey. Food Chem. 2008, 107, 1710–1716. [Google Scholar] [CrossRef]
- Bonina, F.; Saija, A.; Tomaino, A.; Lo Cascio, R.; Rapisarda, P.; Dederen, J.C. In vitro antioxidant activity and in vivo photoprotective effect of a red orange extract. Int. J. Cosmet. Sci. 1998, 20, 331–342. [Google Scholar]
- Tiwari, U.; Cummins, E. Factors influencing levels of phytochemicals in selected fruit and vegetables during pre- and post-harvest food processing operations. Food Res. Int. 2013, 50, 497–506. [Google Scholar] [CrossRef]
- Zou, Z.; Xi, W.; Hu, Y.; Chao Nie, C.; Zhou, Z. Antioxidant activity of Citrus fruits. Food Chem. 2016, 196, 885–896. [Google Scholar] [CrossRef]
- Lo Scalzo, R.; Iannoccari, T.; Summa, C.; Morelli, R.; Rapisarda, P. Effect of thermal treatments on antioxidant and antiradical activity of blood orange juice. Food Chem. 2004, 85, 41–47. [Google Scholar] [CrossRef]
- Arena, E.; Fallico, B.; Maccarone, E. Evaluation of antioxidant capacity of blood orange juices as influenced by constituents, concentration process and storage. Food Chem. 2001, 74, 423–427. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 4–212. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, T.; Horio, F.; Osawa, T. Absorption and metabolism of cyanidin3-O-β-D-glucoside in rats. FEBS Lett. 1999, 449, 179–182. [Google Scholar] [CrossRef]
- Youdim, K.A.; Shukitt-Hale, B.; MacKinnon, S.; Kalt, W.; Joseph, J.A. Polyphenolics enhance red blood cell resistance to oxidative stress: In vitro and in vivo. Biochim. Biophys. Acta 2000, 1523, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Nakagawa, K.; Kudo, M.; Muraishi, K.; Someya, K. Direct intestinal absorption of red fruit anthocyanins, cyanidin-3-glucoside and cyanidin-3, 5-diglucoside, into rats and humans. J. Agric. Food Chem. 1999, 47, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Inaba, H.; Kishi, M.; Tominaga, S.; Hirayama, M.; Tsuda, T. Orally administered delphinidin 3-rutinoside and cyanidin 3-rutinoside are directly absorbed in rats and humans and appear in the blood as the intact forms. J. Agric. Food Chem. 2001, 49, 1546–1551. [Google Scholar] [CrossRef] [PubMed]
- Netzel, M.; Strasse, G.; Carle, E.; Kesenheimer, B.; Janssen, M.; Bitsch, I.; Rechner, A.; Dietrich, H.; Bohm, V.; Bitsch, R. Schwarzer Johannisbeersaft=Functional Food? Lebensmittelchemie 2000, 54, 84–85. [Google Scholar]
- Netzel, M.; Strasse, G.; Janssen, M.; Bitsch, I.; Bitsch, R. Bioactive anthocyanins detected in human urine after ingestion of blackcurrant juice. J. Environ. Pathol. Toxicol. Oncol. 2001, 20, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Muccitelli, H.U.; Sanchez-Moreno, C.; Prior, R.L. Anthocyanins are absorbed in glycated forms in elderly women: A pharmacokinetic study. Am. J. Clin. Nutr. 2001, 73, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, T.; Horio, F.; Osawa, T. The role of anthocyanins as an antioxidant under oxidative stress in rats. Biofactors 2000, 13, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Bourquin, L.D.; Nair, M.G. Degradation products of cyanidin glycosides from tart cherries and their bioactivities. J. Agric. Food Chem. 2001, 49, 4924–4929. [Google Scholar] [CrossRef]
- Vitaglione, P.; Donnarumma, G.; Napolitano, A.; Galvano, F.; Gallo, A.; Scalfi, L.; Fogliano, V. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J. Nutr. 2007, 137, 2043–2048. [Google Scholar] [CrossRef] [Green Version]
- Ciappellano, S.; Brusamolino, A.; Drissner, M.; Galvano, G. Intestinal absorption of phenolic compounds. In Proceedings of the 53rd National Congress of Italian Society of Physiology, Ferrara, Italy, 26–29 September 2002. [Google Scholar]
- Wolffram, S.; Block, M.; Ader, P. Quercetin-3-Glucoside is transported by the glucose carrier SGLT1 across the brush border membrane of rat small intestine. J. Nutr. 2002, 132, 630–635. [Google Scholar] [CrossRef] [Green Version]
- Galvano, F.; La Fauci, L.; Lazzarino, G.; Fogliano, V.; Ritieni, A.; Ciappellano, S.; Battistini, N.C.; Tavazzi, B.; Galvano, G. Cyanidins: Metabolism and biological properties. J. Nutr. Biochem. 2004, 15, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Felgines, C.; Talavera, S.; Texier, O.; Besson, C.; Fogliano, V.; Lamaison, J.L.; La Fauci, L.; Galvano, G.; Rémésy, C.; Galvano, F. Absorption and metabolism of red orange juice anthocyanins in rats. Br. J. Nutr. 2006, 95, 898–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felgines, C.; Texier, O.; Besson, C.; Vitaglione, P.; Lamaison, J.L.; Fogliano, V.; Scalbert, A.; Vanella, L.; Galvano, F. Influence of glucose on cyanidin 3-glucoside absorption in rats. Mol. Nutr. Food Res. 2008, 52, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Walton, M.C.; McGhie, T.K.; Reynolds, G.W.; Hendriks, W.H. The flavonol quercetin-3-glucoside inhibits cyanidin-3-glucoside absorption in vitro. J. Agric. Food Chem. 2006, 54, 4913–4920. [Google Scholar] [CrossRef]
- Faria, A.; Pestana, D.; Azevedo, J.; Martel, F.; de Freitas, V.; Azevedo, I.; Mateus, N.; Calhau, C. Absorption of anthocyanins through intestinal epithelial cells—Putative involvement of GLUT2. Mol. Nutr. Food Res. 2009, 53, 1430–1437. [Google Scholar] [CrossRef]
- Alzaid, F.; Cheung, H.M.; Preedy, V.R.; Sharp, P.A. Regulation of Glucose Transporter Expression in Human Intestinal Caco-2 Cells following Exposure to an Anthocyanin-Rich Berry Extract. PLoS ONE 2013, 8, e78932. [Google Scholar] [CrossRef] [Green Version]
- Han, F.; Yang, P.; Wang, H.; Fernandesd, I.; Mateus, N.; Liu, Y. Digestion and absorption of red grape and wine anthocyanins through the gastrointestinal tract. Trends Food Sci. Technol. 2019, 83, 211–224. [Google Scholar] [CrossRef]
- Zhang, H.; Hassan, Y.I.; Renaud, J.; Liu, R.; Yang, C.; Sun, Y.; Tsao, R. Bioaccessibility, bioavailability, and anti-inflammatory effects of anthocyanins from purple root vegetables using mono-and co-culture cell models. Mol. Nutr. Food Res. 2017, 61, 1600928. [Google Scholar] [CrossRef]
- Zou, T.B.; Feng, D.; Song, G.; Li, H.W.; Tang, H.W.; Ling, W.H. The role of sodium-dependent glucose transporter 1 and glucose transporter 2 in the absorption of cyanidin-3-o-β-glucoside in Caco-2 cells. Nutrients 2014, 6, 4165–4177. [Google Scholar] [CrossRef]
- Baron, G.; Altomare, A.; Regazzoni, L.; Redaelli, V.; Grandi, S.; Riva, A.; Morazzoni, P.; Mazzolari, A.; Carini, M.; Vistoli, G.; et al. Pharmacokinetic profile of bilberry anthocyanins in rats and the role of glucose transporters: LC–MS/MS and computational studies. J. Pharm. Biomed. Anal. 2017, 144, 112–121. [Google Scholar] [CrossRef]
- Talagavadi, V.; Rapisarda, P.; Galvano, F.; Pelicci, P.; Giorgio, M. Cyanidin-3-O-β-glucoside and protocatechuic acid activate AMPK/mTOR/S6K pathway and improve glucose homeostasis in mice. J. Funct. Foods 2016, 21, 338–348. [Google Scholar] [CrossRef]
- Riso, P.; Visioli, F.; Gardana, C.; Grande, S.; Brusamolino, A.; Galvano, F.; Galvano, G.; Porrini, M. Effects of blood orange juice intake on antioxidant bioavailability and on different markers related to oxidative stress. J. Agric. Food Chem. 2005, 53, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Giordano, L.; Coletta, W.; Tamburrelli, C.; D’Imperio, M.; Crescente, M.; Silvestri, C.; Rapisarda, P.; Reforgiato Recupero, G.; De Curtis, A.; Iacoviello, L.; et al. Four-week ingestion of blood orange juice results in measurable anthocyanin urinary levels but does not affect cellular markers related to cardiovascular risk: A randomized cross-over study in healthy volunteers. Eur. J. Nutr. 2012, 51, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Cerletti, C.; Gianfagna, F.; Tamburrelli, C.; De Curtis, A.; D’Imperio, M.; Coletta, W.; Giordano, L.; Lorenzet, R.; Rapisarda, P.; Reforgiato Recupero, G.; et al. Orange juice intake during a fatty meal consumption reduces the postprandial low-grade inflammatory response in healthy subjects. Thromb. Res. 2015, 135, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Hollands, W.J.; Armah, C.N.; Doleman, J.F.; Perez-Moral, N.; Winterbone, M.S.; Kroon, P.A. 4-Week consumption of anthocyanin-rich blood orange juice does not affect LDL-cholesterol or other biomarkers of CVD risk and glycaemia compared with standard orange juice: A randomised controlled trial. Br. J. Nutr. 2018, 119, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Azzini, E.; Venneria, E.; Ciarapica, D.; Foddai, M.; Intorre, F.; Zaccaria, M.; Maiani, F.; Palomba, L.; Barnaba, L.; Tubili, C. Effect of red orange juice consumption on body composition and nutritional status in overweight/obese female: A pilot study. Oxid. Med. Cell. Longev. 2017, 2017, 1672567. [Google Scholar] [CrossRef] [Green Version]
- Buscemi, S.; Rosafio, G.; Arcoleo, G.; Mattina, A.; Canino, B.; Montana, M.; Verga, S.; Rini, G. Effects of red orange juice intake on endothelial function and inflammatory markers in adult subjects with increased cardiovascular risk. Am. J. Clin. Nutr. 2012, 9, 1089–1095. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Lyall, G.K.; Martinez-Blazquez, J.A.; Vallejo, F.; Tomas-Barberan, F.A.; Birch, K.M.; Boesch, C. Blood Orange Juice Consumption Increases Flow-Mediated Dilation in Adults with Overweight and Obesity: A Randomized Controlled Trial. J. Nutr. 2020, 150, 2287–2294. [Google Scholar] [CrossRef]
- Titta, L.; Trinei, M.; Stendardo, M.; Berniakovich, I.; Petroni, K.; Tonelli, C.; Riso, P.; Porrini, M.; Minucci, S.; Pelicci, P.G.; et al. Blood orange juice inhibits fat accumulation in mice. Int. J. Obes. 2010, 34, 578–588. [Google Scholar] [CrossRef] [Green Version]
- Chiechio, S.; Zammataro, M.; Barresi, M.; Amenta, M.; Ballistreri, G.; Fabroni, S.; Rapisarda, P. A Standardized extract prepared from red orange and lemon wastes. Molecules 2021, 26, 4291. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Leuzzi, U.; Gattuso, G. Flavonoid C-glycosides in Citrus juices from southern Italy: Distribution and influence on the antioxidant activity. In Instrumental Methods for the Analysis and Identification of Bioactive Molecole; Jayprakasha, G.K., Patil, B.S., Pellati, F., Eds.; American Chemical Society: Washington, DC, USA, 2014; pp. 189–200. [Google Scholar]
- Ballistreri, G.; Fabroni, S.; Romeo, F.V.; Timpanaro, N.; Amenta, M.; Rapisarda, P. Anthocyanins and Other Polyphenols in Citrus Genus: Biosynthesis, Chemical Profile, and Biological Activity. In Polyphenols in Plants, 2nd ed.; Watson, R., Ed.; Academic Press: London, UK, 2019; pp. 191–215. [Google Scholar]
- Benavente-Garcia, O.; Castillo, J. Update on uses and properties of Citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef] [PubMed]
- Silveira, J.Q.; Dourado, G.K.Z.S.; Cesar, T.B. Red-fleshed sweet orange juice improves the risk factors for metabolic syndrome. Int. J. Food Sci. Nutr. 2015, 66, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; O’Reilly, E.J.; Kay, C.; Sampson, L.; Franz, M.; Forman, J.P.; Curhan, G.; Rimm, E.B. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am. J. Clin. Nutr. 2011, 93, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Bonina, F.P.; Leotta, C.; Scalia, G.; Puglia, C.; Trombetta, D.; Tringali, G.; Roccazzello, A.M.; Rapisarda, P.; Saija, A. Evaluation of oxidative stress in diabetic patients after supplementation with a standardized red orange extract. Diab. Nutr. Metab. 2002, 15, 14–19. [Google Scholar]
- Bonina, F.P.; Puglia, C.; Cimino, F.; Trombetta, D.; Tringali, G.; Roccazzello, A.M.; Insirello, E.; Rapisarda, P.; Saija, A. Oxidative stress in handball players: Effect of supplementation with a red orange extract. Nutr. Res. 2005, 25, 917–924. [Google Scholar] [CrossRef]
- Bonina, F.P.; Puglia, C.; Frasca, G.; Cimino, F.; Trombetta, D.; Tringali, G.; Roccazzello, A.M.; Insiriello, E.; Rapisarda, P.; Saija, A. Protective effects of a standardised red orange extract on air pollution-induced oxidative damage in traffic police officers. Nat. Prod. Res. 2008, 22, 1544–1551. [Google Scholar] [CrossRef]
- Fabroni, S.; Ballistreri, G.; Amenta, M.; Romeo, F.V.; Rapisarda, P. Screening of the anthocyanin profile and in vitro pancreatic lipase inhibition by anthocyanin-containing extracts of fruits, vegetables, legumes and cereals. J. Sci. Food Agric. 2016, 96, 4713–4723. [Google Scholar] [CrossRef] [PubMed]
- Salamone, F.; Li Volti, G.; Titta, L.; Puzzo, L.; Barbagallo, I.; La Delia, F.; Zelber-Sagi, S.; Malaguarnera, M.; Pelicci, P.G.; Giorgio, M.; et al. Moro orange juice prevents fatty liver in mice. World J. Gastroenterol. 2012, 18, 3862–3868. [Google Scholar] [CrossRef]
- Puglia, C.; Offerta, A.; Saija, A.; Trombetta, D.; Cardile, V. Protective effect of red orange extract supplementation against UV-induced skin damages: Photoaging and solar lentigines. J. Cosmet. Dermatol. 2014, 13, 151–157. [Google Scholar] [CrossRef]
- Cardile, V.; Frasca, G.; Rizza, L.; Rapisarda, P.; Bonina, F. Antiinflammatory Effects of a Red Orange Extract in Human Keratinocytes treated with Interferon-gamma and Histamine. Phytother. Res. 2010, 24, 414–418. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fantini, M.; Benvenuto, M.; Masuelli, L.; Vanni Frajese, G.; Tresoldi, I.; Modesti, A.; Bei, R. In Vitro and in Vivo Antitumoral Effects of Combinations of Polyphenols, or Polyphenols and Anticancer Drugs: Perspectives on Cancer Treatment. Int. J. Mol. Sci. 2015, 16, 9236–9282. [Google Scholar] [CrossRef] [PubMed]
- Tsoyi, K.; Park, H.B.; Kim, Y.M.; Chung, J.; Shin, S.C.; Shim, H.J.; Lee, W.S.; Seo, H.G.; Lee, J.H.; Chang, K.C.; et al. Protective Effect of Anthocyanins from Black Soybean Seed Coats on UVB-Induced Apoptotic Cell Death in Vitro and in Vivo. J. Agric. Food Chem. 2008, 56, 10600–10605. [Google Scholar] [CrossRef]
- Jang, C.H.; Lee, I.A.; Ha, Y.R.; Lim, J.; Sung, M.-K.; Lee, S.-J.; Kim, J.-S. PGK1 induction by a hydrogen peroxide treatment is suppressed by antioxidants in human colon carcinoma cells. Biosci. Biotechnol. Biochem. 2008, 72, 1799–1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.-Q.; Kuriyama, S.; Li, Q.; Nagai, M.; Hozawa, A.; Nishino, Y.; Tsuji, I. Citrus consumption and cancer incidence: The Ohsaki cohort study. Int. J. Cancer 2010, 127, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Forester, S.C.; Choy, Y.Y.; Waterhouse, A.L.; Oteiza, P.I. The anthocyanin metabolites gallic acid, 3-O-methylgallic acid, and 2,4,6- trihydroxybenzaldehyde decrease human colon cancer cell viability by regulating pro-oncogenic signals. Mol. Carcinog. 2014, 53, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Ha, U.-S.; Kim, S.-J.; Yoon, B.-I.; Han, D.-S.; Yuk, S.-M.; Kim, S.-W. Anthocyanin extracted from black soybean reduces prostate weight and promotes apoptosis in the prostatic hyperplasia-induced rat model. J. Agric. Food Chem. 2010, 58, 12686–12691. [Google Scholar] [CrossRef]
- Lin, B.W.; Gong, C.C.; Song, H.F.; Cui, Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Gao, J.; Xu, H.; Qian, Y.; Xie, L.; Yu, H.; Qian, B. Citrus fruit intake and lung cancer risk: A meta-analysis of observational studies. Pharm. Res. 2021, 166, 105430. [Google Scholar] [CrossRef]
- Bae, J.-M.; Kim, E.H. Dietary intakes of citrus fruit and risk of gastric cancer incidence: An adaptive meta-analysis of cohort studies. Epidemiol. Health. 2016, 38, e2016034. [Google Scholar] [CrossRef] [Green Version]
- Vingeliene, S.; Chan, D.; Aune, D.; Vieira, A.R.; Polemiti, E.; Stevens, C.; Abar, L.; Rosenblatt, D.N.; Greenwood, D.C.; Norat, T. An update of the WCRF/AICR systematic literature review on esophageal and gastric cancers and citrus fruits intake. Cancer Causes Control 2016, 27, 837–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagiwara, A.; Miyashita, K.; Nakanishi, T.; Sano, M.; Tamano, S.; Kadota, T.; Koda, T.; Nakamura, M.; Imaida, K.; Ito, N.; et al. Pronounced inhibition by a natural anthocyanin, purple corn color, of 2- amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-associated colorectal carcinogenesis in male F344 rats pretreated with 1,2-dimethylhydrazine. Cancer Lett. 2001, 171, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Clifford, M.N. Colonic metabolites of berry polyphenols: The missing link to biological activity? Br. J. Nutr. 2010, 104, 48–66. [Google Scholar] [CrossRef] [Green Version]
- Allegra, V.; Zarbà, C.; La Via, G.; Zarbà, A.S. Why the new orange juice consumption model favors global trade and growth in orange production. Br. Food J. 2019, 121, 1954–1968. [Google Scholar] [CrossRef] [Green Version]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The Case for Anthocyanin Consumption to Promote Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef]







| Citrus sinensis (L.) Osbeck | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak no. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
| Compound | cya 3,5-diglu | del 3-glu | cya 3-soph | cya 3-glu | cya 3-rut | pet 3-glu | del 3-(6M)-glu | peo 3-glu | pet 3-(6M)-glu | cya 3-(6M)-glu | cya 3-(6D)-glu | pel deriv. | peo 3-(6M)-glu | cya deriv. | peo deriv. | |
| Cultivar | Relative composition (%) | Total anthocyanins (mg CGE 100 mL−1) | ||||||||||||||
| Juice | ||||||||||||||||
| ‘Tarocco Rosso’ | 0.69 | 2.64 | 0.29 | 25.40 | 2.04 | 1.22 | 1.37 | 0.81 | 2.18 | 46.81 | 6.31 | 0.76 | 8.66 | 0.29 | 0.53 | 8.11 ± 0.80 a |
| ‘Moro nucellare 58-8D-1′ | 1.49 | 4.43 | 0.65 | 26.81 | 3.73 | 2.06 | 1.92 | 1.58 | 2.50 | 39.86 | 5.80 | 1.80 | 7.11 | 0.37 | 0.61 | 23.52 ± 1.13 d |
| ‘Sanguinello Moscato’ | 0.14 | 8.56 | 1.65 | 45.01 | 0.95 | 0.72 | 0.68 | 1.61 | 1.72 | 22.35 | 12.51 | 0.87 | 1.95 | 1.02 | 0.26 | 17.64 ± 0.63 c |
| Flavedo | ||||||||||||||||
| ‘Moro nucellare 58-8D-1′ | 0.56 | 6.42 | 0.73 | 12.95 | 0.66 | 1.90 | 3.82 | 1.51 | 8.26 | 51.49 | 1.91 | 4.34 | 1.60 | 2.43 | 1.43 | 24.00 ± 0.01 d |
| Flowers—stigma | ||||||||||||||||
| ‘Moro nucellare 58-8D-1′ | 0.11 | 2.53 | 0.77 | 10.30 | 0.66 | 4.62 | 3.01 | 1.43 | 3.64 | 36.73 | 6.29 | 8.78 | 15.30 | 0.44 | 5.41 | 12.23 ± 1.18 b |
| Diet Supplementation | Dosage | Duration | Health Effects | References |
|---|---|---|---|---|
| Red orange juice | 600 mL/day | 21 days |
| Riso et al. [107] |
| 1 L/day | 4 weeks |
| Giordano et al. [108] | |
| 1 L | A fatty meal that lasted 15 min | Short-term effects (2 h after meal):
| Cerletti et al. [109] | |
| 500 mL/day | 28 days |
| Hollands et al. [110] | |
| 500 mL/day | 12 weeks |
| Azzini et al. [111] | |
| 500 mL/day | 2 periods of 7 d each with a 3-d interval | in subjects with high cardiovascular risk:
| Buscemi et al. [112] | |
| 200 mL twice daily | 2 weeks with a washout period of 1 week |
| Li et al. [113] | |
| As hydrating medium instead of water | 12 weeks |
| Titta et al. [114] | |
| Red orange extract | 120 mg/kg/day of total anthocyanins | 8 weeks |
| Chiechio et al. [115] |
| Author(s),. Year | Risk Factor | Effect of Citrus Anthocyanins | Disease |
|---|---|---|---|
| Grosso et al., 2013 [22]; Buscemi et al., 2012 [112]; Silveira et al., 2015 [119]; Cassidy et al., 2011 [120]. | Blood pressure | Decreased vascular inflammation | Heart disease (atherosclerosis, high systolic blood pressure, high level of chlolesterol, hypertension, ischemic heart) |
| Grosso et al., 2013 [22]; Silveira et al., 2015 [119]. | Cholesterol | Help cholesterol level by raising HDL and lowering LDL cholesterol | Heart disease (high level of LDL cholesterol, stroke) |
| Grosso et al., 2013 [22]; Bonina et al., 2002, 2005, 2008 [121,122,123]. | Oxidation | Decrease oxidation | Heart disease (atherosclerosis, lipid oxidation, oxidative stress) |
| Grosso et al., 2013 [22]; Cerletti et al., 2015 [109]. | Inflammation | Decrease inflammation | Heart disease (atherosclerosis, oxidative stress, vascular stiffness), obesity (high level of abdominal fat) |
| Talagavadi et al., 2016 [106]; Titta et al., 2010 [114]; Fabroni et al., 2016 [124]; Salamone et al., 2012 [125]. | Abdominal fat | Enhanced lipase enzyme activity | Obesity (high blood lipid levels), fatty liver (hepatic steatosis), type 2 diabetes (uncontrolled oxidation of lipids), metabolic syndrome (abdominal obesity, high blood sugar, high cholesterol, hypertension) |
| Grosso et al., 2013 [22]; Silveira et al., 2015 [119]. | Blood levels of glucose | Improved insulin sensitivity | Type 2 diabetes (oxidative damage, high glucose level, high blood pressure) |
| Bonina et al., 1998 [82]; Puglia et al., 2014 [126]; Cardile et al., 2010 [127]. | UV radiations | Photoprotective, anti-ageing | Oxidative damage (skin rash, photo-oxidative skin lesions, allergic contact dermatitis, psoriasis, atopic dermatitis) |
| Author(s), Year | Risk Factor | Effect of Citrus Anthocyanins | Disease |
|---|---|---|---|
| Tsoyi et al., 2008 [130] | UVB radiations | Photoprotective, | Photocarcinogenesis, apoptotic cell death |
| Jang et al., 2008 [131] | Intracellular oxidative damage | Anti-carcinogenic | Colon Carcinoma, angiogenesis |
| Li et al., 2010 [132] | Inflammation | Reduced risk of prostate or pancreatic cancer | Prostatic or pancreatic cancer |
| Forester et al., 2014 [133] | Inflammation | Decreasing cell viability, cell cycle arrest and apoptosis | Colon cancer |
| Jang et al., 2010 [134] | Inflammation | Reduce prostatic hyperplasia | Prostatic cancer |
| Grosso et al., 2013 [22] | Cell mutation | Anti-carcinogenic, anti mutagenic | Colonic adenocarcinoma, melanoma, vulva carcinoma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapisarda, P.; Amenta, M.; Ballistreri, G.; Fabroni, S.; Timpanaro, N. Distribution, Antioxidant Capacity, Bioavailability and Biological Properties of Anthocyanin Pigments in Blood Oranges and Other Citrus Species. Molecules 2022, 27, 8675. https://doi.org/10.3390/molecules27248675
Rapisarda P, Amenta M, Ballistreri G, Fabroni S, Timpanaro N. Distribution, Antioxidant Capacity, Bioavailability and Biological Properties of Anthocyanin Pigments in Blood Oranges and Other Citrus Species. Molecules. 2022; 27(24):8675. https://doi.org/10.3390/molecules27248675
Chicago/Turabian StyleRapisarda, Paolo, Margherita Amenta, Gabriele Ballistreri, Simona Fabroni, and Nicolina Timpanaro. 2022. "Distribution, Antioxidant Capacity, Bioavailability and Biological Properties of Anthocyanin Pigments in Blood Oranges and Other Citrus Species" Molecules 27, no. 24: 8675. https://doi.org/10.3390/molecules27248675
APA StyleRapisarda, P., Amenta, M., Ballistreri, G., Fabroni, S., & Timpanaro, N. (2022). Distribution, Antioxidant Capacity, Bioavailability and Biological Properties of Anthocyanin Pigments in Blood Oranges and Other Citrus Species. Molecules, 27(24), 8675. https://doi.org/10.3390/molecules27248675