Kinetics of Carotenoids Degradation during the Storage of Encapsulated Carrot Waste Extracts
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Reagents and Solvents
3.2. Samples
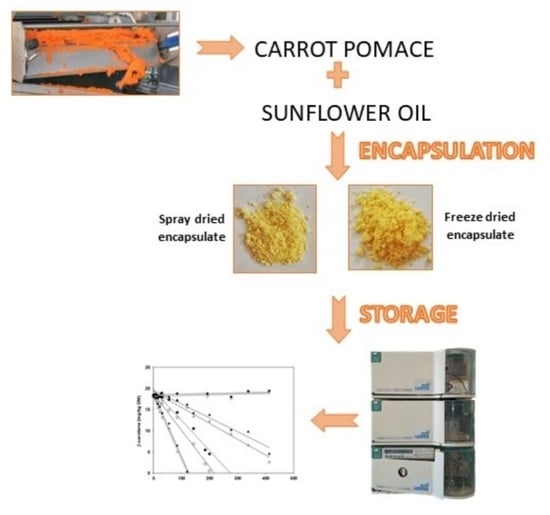
3.3. Carrot Waste Extraction and Encapsulates Preparation
3.4. Storage
3.5. Chemical Analysis
3.6. Degradation Kinetics Modelling
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Awasthi, S.; Awasthi, A. Role of vitamin A in child health and nutrition. Clin. Epidemiol. Glob. Health 2020, 8, 1039–1042. [Google Scholar] [CrossRef]
- Stephensen, C.B.; Lietz, G. Vitamin A in resistance to and recovery from infection: Relevance to SARS-CoV-2. Br. J. Nutr. 2021, 126, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Šeregelj, V.; Vulić, J.; Ćetković, G.; Čanadanović-Brunet, J.; Tumbas Šaponjac, V.; Stajčić, S. Natural bioactive compounds in carrot waste for food applications and health benefits. In Bioactive Natural Products; Atta-ur-Rahman, Ed.; Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; Volume 67, pp. 307–344. [Google Scholar]
- Klettenhammer, S.; Ferrentino, G.; Zendehbad, H.S.; Morozova, K.; Scampicchio, M. Microencapsulation of linseed oil enriched with carrot pomace extracts using Particles from Gas Saturated Solutions (PGSS) process. J. Food Eng. 2022, 312, 110746. [Google Scholar] [CrossRef]
- Polat, S.; Guclu, G.; Kelebek, H.; Keskin, M.; Selli, S. Comparative elucidation of colour, volatile and phenolic profiles of black carrot (Daucus carota L.) pomace and powders prepared by five different drying methods. Food Chem. 2022, 369, 130941. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fabiano-Tixier, A.S.; Tomao, V.; Cravotto, G.; Chemat, F. Green ultrasound-assisted extraction of carotenoids based on the bio-refinery concept using sunflower oil as an alternative solvent. Ultrason. Sonochem. 2013, 20, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Sachindra, N.M.; Mahendrakar, N.S. Process optimization for extraction of carotenoids from shrimp waste with vegetable oils. Bioresour. Technol. 2005, 96, 1195–1200. [Google Scholar] [CrossRef]
- Tiwari, S.; Upadhyay, N.; Malhotra, R. Three-way ANOVA for emulsion of carotenoids extracted in flaxseed oil from carrot bio-waste. Waste Manag. 2021, 121, 67–76. [Google Scholar] [CrossRef]
- Liu, J.; Bi, J.; Liu, X.; Zhang, B.; Wu, X.; Wellala, C.K.D.; Zhang, B. Effects of high pressure homogenization and addition of oil on the carotenoid bioaccessibility of carrot juice. Food Funct. 2019, 22, 458–468. [Google Scholar] [CrossRef]
- Reif, C.; Arrigoni, E.; Baumgartner, D.; Schärer, H.; Nising, A.B.; Hurrell, R.F. Adaption of an in vitro digestion method to evaluate carotenoid accessibility from vegetables. Acta Hortic. 2009, 1040, 255–260. [Google Scholar] [CrossRef]
- Colle, I.J.P.; Lemmens, L.; Knockaert, G.; Van Loey, A.; Hendrickx, M. Carotene degradation and isomerization during thermal processing: A review on the kinetic aspects. Crit. Rev. Food Sci. Nutr. 2016, 56, 1844–1855. [Google Scholar] [CrossRef]
- Eun, J.; Maruf, A.; Das, P.R.; Nam, S. A review of encapsulation of carotenoids using spray drying and freeze drying. Crit. Rev. Food Sci. Nutr. 2020, 60, 3547–3572. [Google Scholar] [CrossRef] [PubMed]
- Ćetković, G.; Šeregelj, V.; Brandolini, A.; Čanadanović-Brunet, J.; Tumbas Šaponjac, V.; Vulić, J.; Šovljanski, O.; Četojević-Simin, D.; Škrobot, D.; Mandić, A.; et al. Composition, texture, sensorial quality, and biological activity after in vitro digestion of durum wheat pasta enriched with carrot waste extract encapsulates. Int. J. Food Sci. Nutr. 2022, 11, 1130. [Google Scholar] [CrossRef] [PubMed]
- Šeregelj, V.; Škrobot, D.; Kojić, I.; Pezo, L.; Šovljanski, O.; Tumbas Šaponjac, V.; Vulić, J.; Hidalgo, A.; Brandolini, A.; Čanadanović-Brunet, J.; et al. Quality and sensory profile of durum wheat pasta enriched with carrot waste encapsulates. Foods 2022, 11, 1130. [Google Scholar] [CrossRef] [PubMed]
- Šeregelj, V.; Ćetković, G.; Čanadanović-Brunet, J.; Tumbas Šaponjac, V.; Vulić, J.; Lević, S.; Nedović, V.; Brandolini, A.; Hidalgo, A. Encapsulation of carrot waste extract by freeze and spray drying techniques: An optimization study. LWT 2021, 138, 110696. [Google Scholar] [CrossRef]
- Marx, M.; Stuparic, M.; Schieber, A.; Carle, R. Effects of thermal processing on trans–cis-isomerization of β-carotene in carrot juices and carotene-containing preparations. Food Chem. 2003, 83, 609–617. [Google Scholar] [CrossRef]
- Imsic, M.; Winkler, S.; Tomkins, B.; Jones, R. Effect of storage and cooking on β-carotene isomers in carrots (Daucus carota L. cv. ‘Stefano’). J. Agric. Food Chem. 2010, 58, 5109–5113. [Google Scholar] [CrossRef]
- Fratianni, A.; Niro, S.; Messia, M.C.; Panfili, G.; Marra, F.; Cinquanta, L. Evaluation of carotenoids and furosine content in air dried carrots and parsnips pre-treated with pulsed electric field (PEF). Eur. Food Res. Technol. 2019, 245, 2529–2537. [Google Scholar] [CrossRef]
- Qiu, D.; Chen, Z.-R.; Li, H.-R. Density functional theory study on thermal isomerization of β-carotene. J. Mol. Struc. THEOCHEM 2008, 865, 44–48. [Google Scholar] [CrossRef]
- Mínguez-Mosquera, M.I.; Hornero-Méndez, D.; Pérez-Gálvez, A. Carotenoids and provitamin A in functional foods. In Methods of Analysis for Functional Foods and Nutraceuticals, 2nd ed.; Hurst, W.J., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 277–336. [Google Scholar] [CrossRef]
- Schieber, A.; Carle, R. Occurrence of carotenoid cis-isomers in food: Technological, analytical, and nutritional implications. Trends Food Sci. Technol. 2005, 16, 416–422. [Google Scholar] [CrossRef]
- Reiter, M.; Stuparić, M.; Neidhart, S.; Carle, R. The role of process technology in carrot juice cloud stability. LWT 2003, 36, 165–172. [Google Scholar] [CrossRef]
- Negi, P.S.; Roy, S.K. The effect of blanching on quality attributes of dehydrated carrots during long-term storage. Eur. Food Res. Technol. 2001, 212, 445–448. [Google Scholar] [CrossRef]
- Chen, J.; Li, F.; Li, Z.; McClements, D.J.; Xiao, H. Encapsulation of carotenoids in emulsion-based delivery systems: Enhancement of β-carotene water-dispersibility and chemical stability. Food Hydrocoll. 2017, 69, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Wei, Q.; Wang, X.; Li, D.; Liu, C.; Zhang, M.; Meng, L. Degradation of carotenoids in dehydrated pumpkins as affected by different storage conditions. Food Res. Int. 2018, 107, 130–136. [Google Scholar] [CrossRef]
- Haas, K.; Obernberger, J.; Zehetner, E.; Kiesslich, A.; Volkert, M.; Jaeger, H. Impact of powder particle structure on the oxidation stability and color of encapsulated crystalline and emulsified carotenoids in carrot concentrate powders. J. Food Eng. 2019, 263, 398–408. [Google Scholar] [CrossRef]
- Kha, T.C.; Nguyen, M.H.; Roach, P.D.; Stathopoulos, C.E. A storage study of encapsulated gac (Momordica cochinchinensis) oil powder and its fortification into foods. Food Bioprod. Process. 2015, 96, 113–125. [Google Scholar] [CrossRef]
- Lee, W.J.; Tan, C.P.; Sulaiman, R.; Hee, Y.Y.; Chong, G.H. Storage stability and degradation kinetics of bioactive compounds in red palm oil microcapsules produced with solution-enhanced dispersion by supercritical carbon dioxide: A comparison with the spray-drying method. Food Chem. 2020, 304, 125427. [Google Scholar] [CrossRef] [PubMed]
- Tupuna, D.S.; Paese, K.; Stanisçuaski Guterres, S.S.; Jablonski, A.; Hickmann Flôres, S.; de Oliveira Rios, A. Encapsulation efficiency and thermal stability of norbixin microencapsulated by spray-drying using different combinations of wall materials. Ind. Crops Prod. 2018, 111, 846–855. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Roach, P.D.; Golding, J.B.; Parks, S.E.; Nguyen, M.H. Encapsulation of carotenoid-rich oil from Gac peel: Optimisation of the encapsulating process using a spray drier and the storage stability of encapsulated powder. Powder Technol. 2019, 344, 373–379. [Google Scholar] [CrossRef]
- Lima, P.M.; Dacanal, G.C.; Pinho, L.S.; Pérez-Cordoba, L.J.; Thomazini, M.; Freitas Moraes, I.C.; Favaro-Trindade, C.S. Production of a rich-carotenoid colorant from pumpkin peels using oil-in-water emulsion followed by spray drying. Food Res. Int. 2021, 148, 110627. [Google Scholar] [CrossRef]
- Soukoulis, C.; Cambier, S.; Hoffmann, L.; Bohn, T. Chemical stability and bioaccessibility of β-carotene encapsulated in sodium alginate o/w emulsions: Impact of Ca2+ mediated gelation. Food Hydrocoll. 2016, 57, 301–310. [Google Scholar] [CrossRef]
- Soukoulis, C.; Tsevdou, M.; Andre, C.M.; Cambier, S.; Yonekura, L.; Taoukis, P.S.; Hoffmann, L. Modulation of chemical stability and in vitro bioaccessibility of beta-carotene loaded in kappa-carrageenan oil-in-gel emulsions. Food Chem. 2017, 220, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Wagner, L.A.; Warthesen, J.J. Stability of spray-dried encapsulated carrot carotenes. J. Food Sci. 1995, 60, 1048–1053. [Google Scholar] [CrossRef]
- Desobry, S.A.; Netto, F.M.; Labuza, T.P. Influence of maltodextrin systems at an equivalent 25DE on encapsulated β-carotene loss during storage. J. Food Process. Preserv. 1999, 23, 39–55. [Google Scholar] [CrossRef]
- Lim, A.S.L.; Griffin, C.; Roos, Y.H. Stability and loss kinetics of lutein and β-carotene encapsulated in freeze-dried emulsions with layered interface and trehalose as glass former. Food Res. Int. 2014, 62, 403–409. [Google Scholar] [CrossRef]
- Przybysz, M.A.; Szterk, A.; Symoniuk, E.; Gąszczyk, M.; Dłużewska, E. α- and β-carotene stability during storage of microspheres obtained from spray-dried microencapsulation technology. Pol. J. Food Nutr. Sci. 2018, 68, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, C.; Cui, B.; Wang, M.; Fu, H.; Wang, Y. Carotenoid-enriched oil preparation and stability analysis during storage: Influence of oils’ chain length and fatty acid saturation. LWT 2021, 151, 112163. [Google Scholar] [CrossRef]
- Lavelli, V.; Sereikaitè, J. Kinetic study of encapsulated carotene degradation in dried systems: A review. Foods 2022, 11, 437. [Google Scholar] [CrossRef]
- Desobry, S.A.; Netto, F.M.; Labuza, T.P. Comparison of spray-drying, drum-drying and freeze-drying for β-carotene encapsulation and preservation. J. Food Sci. 1997, 62, 1158–1162. [Google Scholar] [CrossRef]
- Koca, N.; Burdurlu, H.S.; Karadeniz, F. Kinetics of colour changes in dehydrated carrots. J. Food Eng. 2007, 78, 449–455. [Google Scholar] [CrossRef]
- Hidalgo, A.; Brandolini, A. Kinetics of carotenoids degradation during the storage of einkorn (Triticum monococcum L. ssp. monococcum) and bread wheat (Triticum aestivum L. ssp. aestivum) flours. J. Agric. Food Chem. 2008, 56, 11300–11305. [Google Scholar] [CrossRef]


| α-Carotene | β-Carotene | cis-β-Carotene | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| °C | r | k | t1/2 | r | k | t1/2 | r | k | t1/2 | |
| Zero-order | ||||||||||
| FDE | 4 | 0.21 | 0.000 | 0.55 | 0.000 | 0.41 | 0.001 | |||
| 21 | 0.98 | 0.009 | 319.1 | 0.98 | 0.033 | 281.2 | 0.81 | 0.007 | 366.7 | |
| 30 | 0.99 | 0.019 | 141.1 | 0.98 | 0.071 | 129.2 | 0.95 | 0.019 | 130.8 | |
| 37 | 0.99 | 0.045 | 61.3 | 1.00 | 0.149 | 61.9 | 0.89 | 0.058 | 43.1 | |
| SDE | 4 | 0.56 | 0.001 | 0.44 | 0.000 | 0.78 | 0.004 | |||
| 21 | 0.99 | 0.010 | 265.2 | 0.99 | 0.037 | 241.3 | 0.99 | 0.013 | 256.7 | |
| 30 | 1.00 | 0.026 | 103.9 | 0.99 | 0.087 | 104.2 | 0.98 | 0.036 | 94.7 | |
| 37 | 0.99 | 0.045 | 60.7 | 1.00 | 0.148 | 61.1 | 0.94 | 0.064 | 53.1 | |
| First-order | ||||||||||
| FDE | 4 | 0.20 | 0.000 | 0.54 | 0.000 | 0.39 | 0.000 | |||
| 21 | 0.96 | 0.003 | 0.94 | 0.003 | 0.88 | 0.002 | ||||
| 30 | 0.97 | 0.006 | 0.96 | 0.007 | 0.93 | 0.006 | ||||
| 37 | 0.87 | 0.031 | 0.88 | 0.029 | 0.91 | 0.029 | ||||
| SDE | 4 | 0.55 | 0.000 | 0.43 | 0.000 | 0.76 | 0.001 | |||
| 21 | 0.95 | 0.004 | 0.93 | 0.004 | 0.94 | 0.004 | ||||
| 30 | 0.92 | 0.014 | 0.91 | 0.014 | 0.90 | 0.014 | ||||
| 37 | 0.93 | 0.026 | 0.91 | 0.029 | 0.91 | 0.027 | ||||
| Ea (kJ/mol) | z (°C) | Ea (kJ/mol) | z (°C) | |
|---|---|---|---|---|
| FDE | SDE | |||
| α-carotene | 79.2 | 22.11 | 71.7 | 24.42 |
| β-carotene | 72.8 | 24.05 | 66.6 | 26.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šeregelj, V.; Estivi, L.; Brandolini, A.; Ćetković, G.; Tumbas Šaponjac, V.; Hidalgo, A. Kinetics of Carotenoids Degradation during the Storage of Encapsulated Carrot Waste Extracts. Molecules 2022, 27, 8759. https://doi.org/10.3390/molecules27248759
Šeregelj V, Estivi L, Brandolini A, Ćetković G, Tumbas Šaponjac V, Hidalgo A. Kinetics of Carotenoids Degradation during the Storage of Encapsulated Carrot Waste Extracts. Molecules. 2022; 27(24):8759. https://doi.org/10.3390/molecules27248759
Chicago/Turabian StyleŠeregelj, Vanja, Lorenzo Estivi, Andrea Brandolini, Gordana Ćetković, Vesna Tumbas Šaponjac, and Alyssa Hidalgo. 2022. "Kinetics of Carotenoids Degradation during the Storage of Encapsulated Carrot Waste Extracts" Molecules 27, no. 24: 8759. https://doi.org/10.3390/molecules27248759
APA StyleŠeregelj, V., Estivi, L., Brandolini, A., Ćetković, G., Tumbas Šaponjac, V., & Hidalgo, A. (2022). Kinetics of Carotenoids Degradation during the Storage of Encapsulated Carrot Waste Extracts. Molecules, 27(24), 8759. https://doi.org/10.3390/molecules27248759