Polymeric Nanoparticles with Embedded Eu(III) Complexes as Molecular Probes for Temperature Sensing
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Thermosensitive Eu(III) Complexes
2.2. Photophysical Properties of Eu(III) Complexes
2.3. Synthesis of Latex Nanoparticles (NPs)
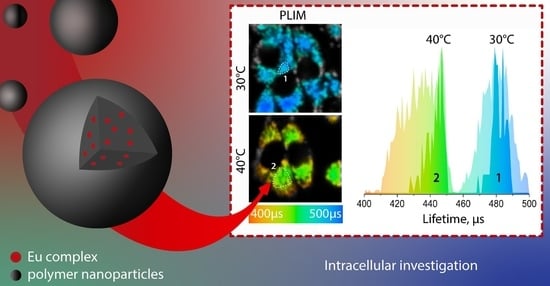
2.4. Cell Experiments
2.4.1. Cytotoxicity and Localization of Probe in Cells
2.4.2. PLIM Experiments
2.5. Modification of Latex NPs’ Surface
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bai, T.; Gu, N. Micro/Nanoscale Thermometry for Cellular Thermal Sensing. Small 2016, 12, 4590–4610. [Google Scholar] [CrossRef] [PubMed]
- Del Rosal, B.; Ximendes, E.; Rocha, U.; Jaque, D. In Vivo Luminescence Nanothermometry: From Materials to Applications. Adv. Opt. Mater. 2017, 5, 1600508. [Google Scholar] [CrossRef]
- Nakano, M.; Nagai, T. Thermometers for Monitoring Cellular Temperature. J. Photochem. Photobiol. C Photochem. Rev. 2017, 30, 2–9. [Google Scholar] [CrossRef]
- Dramićanin, M. Luminescence: The Basics, Methods, and Instrumentation. In Luminescence Thermometry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 33–61. [Google Scholar]
- Dramićanin, M.D. Trends in Luminescence Thermometry. J. Appl. Phys. 2020, 128, 040902. [Google Scholar] [CrossRef]
- Ogle, M.M.; Smith McWilliams, A.D.; Jiang, B.; Martí, A.A. Latest Trends in Temperature Sensing by Molecular Probes. ChemPhotoChem 2020, 4, 255–270. [Google Scholar] [CrossRef]
- Wang, X.; Wolfbeis, O.S.; Meier, R.J. Luminescent Probes and Sensors for Temperature. Chem. Soc. Rev. 2013, 42, 7834–7869. [Google Scholar] [CrossRef]
- Qin, T.; Liu, B.; Zhu, K.; Luo, Z.; Huang, Y.; Pan, C.; Wang, L. Organic Fluorescent Thermometers: Highlights from 2013 to 2017. TrAC Trends Anal. Chem. 2018, 102, 259–271. [Google Scholar] [CrossRef]
- Okabe, K.; Sakaguchi, R.; Shi, B.; Kiyonaka, S. Intracellular Thermometry with Fluorescent Sensors for Thermal Biology. Pflug. Arch. 2018, 470, 717–731. [Google Scholar] [CrossRef]
- Li, R.; Xu, F.-F.; Gong, Z.-L.; Zhong, Y.-W. Thermo-Responsive Light-Emitting Metal Complexes and Related Materials. Inorg. Chem. Front. 2020, 7, 3258–3281. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Yu, Q.; Wei, H.; Liu, S.; Zhao, Q.; Huang, W. Long-Lived Emissive Probes for Time-Resolved Photoluminescence Bioimaging and Biosensing. Chem. Rev. 2018, 118, 1770–1839. [Google Scholar] [CrossRef]
- Rocha, J.; Brites, C.D.S.; Carlos, L.D. Lanthanide Organic Framework Luminescent Thermometers. Chem. Eur. J. 2016, 22, 14782–14795. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.T.; Khan, N.Z.; Mao, J.; Qiu, L.; Wei, X.; Chen, Y.; Khan, S.A. Lanthanide and Transition Metals Doped Materials for Non-Contact Optical Thermometry with Promising Approaches. Mater. Today Chem. 2022, 24, 100903. [Google Scholar] [CrossRef]
- Wang, C.; Jin, Y.; Zhang, R.; Yao, Q.; Hu, Y. A Review and Outlook of Ratiometric Optical Thermometer Based on Thermally Coupled Levels and Non-Thermally Coupled Levels. J. Alloys Compd. 2022, 894, 162494. [Google Scholar] [CrossRef]
- Ansari, A.A.; Parchur, A.K.; Nazeeruddin, M.K.; Tavakoli, M.M. Luminescent Lanthanide Nanocomposites in Thermometry: Chemistry of Dopant Ions and Host Matrices. Coord. Chem. Rev. 2021, 444, 214040. [Google Scholar] [CrossRef]
- Dramićanin, M. Biomedical Applications of Luminescence Thermometry. In Luminescence Thermometry; Dramićanin, M., Ed.; Woodhead Publishing Series in Electronic and Optical Materials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 235–250. [Google Scholar]
- Li, H.; Zhao, Y.; Kolesnikov, I.; Xu, S.; Chen, L.; Bai, G. Multifunctional Rare Earth Ions-Doped Ba2LaF7 Nanocrystals for Simultaneous Temperature Sensing and Photothermal Therapy. J. Alloys Compd. 2023, 931, 167535. [Google Scholar] [CrossRef]
- Savchuk, O.A.; Carvajal, J.J.; Haro-Gonzalez, P.; Aguiló, M.; Díaz, F. Luminescent Nanothermometry Using Short-Wavelength Infrared Light. J. Alloys Compd. 2018, 746, 710–719. [Google Scholar] [CrossRef]
- Alkahtani, M.; Jiang, L.; Brick, R.; Hemmer, P.; Scully, M. Nanometer-Scale Luminescent Thermometry in Bovine Embryos. Opt. Lett. 2017, 42, 4812–4815. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Lima, P.P.; Silva, N.J.O.; Millán, A.; Amaral, V.S.; Palacio, F.; Carlos, L.D. Thermometry at the Nanoscale. Nanoscale 2012, 4, 4799–4829. [Google Scholar] [CrossRef] [Green Version]
- Bustamante, N.; Ielasi, G.; Bedoya, M.; Orellana, G. Optimization of Temperature Sensing with Polymer-Embedded Luminescent Ru(II) Complexes. Polymers 2018, 10, 234. [Google Scholar] [CrossRef] [Green Version]
- Santoro, S.; Sebastian, V.; Moro, A.J.; Portugal, C.A.M.; Lima, J.C.; Coelhoso, I.M.; Crespo, J.G.; Mallada, R. Development of Fluorescent Thermoresponsive Nanoparticles for Temperature Monitoring on Membrane Surfaces. J. Colloid Interface Sci. 2017, 486, 144–152. [Google Scholar] [CrossRef]
- Fischer, L.H.; Stich, M.I.J.; Wolfbeis, O.S.; Tian, N.; Holder, E.; Schäferling, M. Red- and Green-Emitting Iridium(III) Complexes for a Dual Barometric and Temperature-Sensitive Paint. Chem. Eur. J. 2009, 15, 10857–10863. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.H.; Karakus, C.; Meier, R.J.; Risch, N.; Wolfbeis, O.S.; Holder, E.; Schäferling, M. Referenced Dual Pressure- and Temperature-Sensitive Paint for Digital Color Camera Read Out. Chem. Eur. J. 2012, 18, 15706–15713. [Google Scholar] [CrossRef] [PubMed]
- Karakus, C.; Fischer, L.H.; Schmeding, S.; Hummel, J.; Risch, N.; Schäferling, M.; Holder, E. Oxygen and Temperature Sensitivity of Blue to Green to Yellow Light-Emitting Pt(II) Complexes. Dalton Trans. 2012, 41, 9623–9632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gálico, D.A.; Mazali, Í.O.; Sigoli, F.A. A Highly Sensitive Luminescent Ratiometric Thermometer Based on Europium(III) and Terbium(III) Benzoylacetonate Complexes Chemically Bonded to Ethyldiphenylphosphine Oxide Functionalized Polydimethylsiloxane. New J. Chem. 2018, 42, 18541–18549. [Google Scholar] [CrossRef]
- Werts, M.H.V. Making Sense of Lanthanide Luminescence. Sci. Prog. 2005, 88, 101–131. [Google Scholar] [CrossRef]
- D’Aléo, A.; Picot, A.; Baldeck, P.L.; Andraud, C.; Maury, O. Design of Dipicolinic Acid Ligands for the Two-Photon Sensitized Luminescence of Europium Complexes with Optimized Cross-Sections. Inorg. Chem. 2008, 47, 10269–10279. [Google Scholar] [CrossRef]
- Dramićanin, M. Lanthanide and Transition Metal Ion Doped Materials for Luminescence Temperature Sensing. In Luminescence Thermometry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 113–157. [Google Scholar]
- Cotton, S. Electronic and Magnetic Properties of the Lanthanides. In Lanthanide and Actinide Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2006; pp. 61–87. [Google Scholar]
- Shakirova, J.R.; Shevchenko, N.N.; Baigildin, V.A.; Chelushkin, P.S.; Khlebnikov, A.F.; Tomashenko, O.A.; Solomatina, A.I.; Starova, G.L.; Tunik, S.P. Eu-Based Phosphorescence Lifetime Polymer Nanothermometer: A Nanoemulsion Polymerization Approach to Eliminate Quenching of Eu Emission in Aqueous Media. ACS Appl. Polym. Mater. 2020, 2, 537–547. [Google Scholar] [CrossRef]
- Galenko, E.E.; Novikov, M.S.; Shakirova, F.M.; Shakirova, J.R.; Kornyakov, I.V.; Bodunov, V.A.; Khlebnikov, A.F. Isoxazole Strategy for the Synthesis of 2,2′-Bipyridine Ligands: Symmetrical and Unsymmetrical 6,6′-Binicotinates, 2,2′-Bipyridine-5-Carboxylates, and Their Metal Complexes. J. Org. Chem. 2019, 84, 3524–3536. [Google Scholar] [CrossRef]
- Karhunen, U.; Jaakkola, L.; Wang, Q.; Lamminmäki, U.; Soukka, T. Luminescence Switching by Hybridization-Directed Mixed Lanthanide Complex Formation. Anal. Chem. 2010, 82, 751–754. [Google Scholar] [CrossRef]
- Lo, W.-S.; Kwok, W.-M.; Law, G.-L.; Yeung, C.-T.; Chan, C.T.-L.; Yeung, H.-L.; Kong, H.-K.; Chen, C.-H.; Murphy, M.B.; Wong, K.-L.; et al. Impressive Europium Red Emission Induced by Two-Photon Excitation for Biological Applications. Inorg. Chem. 2011, 50, 5309–5311. [Google Scholar] [CrossRef]
- Englman, R.; Jortner, J. The Energy Gap Law for Radiationless Transitions in Large Molecules. Mol. Phys. 1970, 18, 145–164. [Google Scholar] [CrossRef]
- Chelushkin, P.S.; Shakirova, J.R.; Kritchenkov, I.S.; Baigildin, V.A.; Tunik, S.P. Phosphorescent NIR Emitters for Biomedicine: Applications, Advances and Challenges. Dalton Trans. 2022, 51, 1257–1280. [Google Scholar] [CrossRef] [PubMed]
- Baigildin, V.; Pankova, G.; Evseeva, T.; Lavrov, N.; Shirokova, I.; Vaganov, G.; Shevchenko, N. Methyl Methacrylate Particles with Amino Groups on the Surface: Colloid Stability and Sorption of Biologically Active Substances. J. Dispers. Sci. Technol. 2017, 38, 1570–1577. [Google Scholar] [CrossRef]
- Su, X.; Wen, Y.; Yuan, W.; Xu, M.; Liu, Q.; Huang, C.; Li, F. Lifetime-Based Nanothermometry in Vivo with Ultra-Long-Lived Luminescence. Chem. Commun. 2020, 56, 10694–10697. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Kam, C.; Chou, T.Y.; Wu, M.-Y.; Zhao, X.; Chen, S. A Simple yet Effective AIE-Based Fluorescent Nano-Thermometer for Temperature Mapping in Living Cells Using Fluorescence Lifetime Imaging Microscopy. Nanoscale Horiz. 2020, 5, 488–494. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liang, S.; Mei, M.; Zhao, Q.; She, G.; Shi, W.; Mu, L. Sensitive and Stable Thermometer Based on the Long Fluorescence Lifetime of Au Nanoclusters for Mitochondria. Anal. Chem. 2021, 93, 15072–15079. [Google Scholar] [CrossRef]
- Itoh, H.; Arai, S.; Sudhaharan, T.; Lee, S.-C.; Chang, Y.-T.; Ishiwata, S.; Suzuki, M.; Lane, E.B. Direct Organelle Thermometry with Fluorescence Lifetime Imaging Microscopy in Single Myotubes. Chem. Commun. 2016, 52, 4458–4461. [Google Scholar] [CrossRef] [Green Version]
- Allison, S.W.; Gillies, G.T.; Rondinone, A.J.; Cates, M.R. Nanoscale Thermometry via the Fluorescence of YAG:Ce Phosphor Particles: Measurements from 7 to 77 °C. Nanotechnology 2003, 14, 859–863. [Google Scholar] [CrossRef]
- Baleizão, C.; Nagl, S.; Borisov, S.M.; Schäferling, M.; Wolfbeis, O.S.; Berberan-Santos, M.N. An Optical Thermometer Based on the Delayed Fluorescence of C70. Chem. Eur. J. 2007, 13, 3643–3651. [Google Scholar] [CrossRef]
- Peng, H.; Stich, M.I.J.; Yu, J.; Sun, L.; Fischer, L.H.; Wolfbeis, O.S. Luminescent Europium(III) Nanoparticles for Sensing and Imaging of Temperature in the Physiological Range. Adv. Mater. 2010, 22, 716–719. [Google Scholar] [CrossRef]
- Shang, L.; Stockmar, F.; Azadfar, N.; Nienhaus, G.U. Intracellular Thermometry by Using Fluorescent Gold Nanoclusters. Angew. Chem. Int. Ed. 2013, 52, 11154–11157. [Google Scholar] [CrossRef] [PubMed]
- Okabe, K.; Inada, N.; Gota, C.; Harada, Y.; Funatsu, T.; Uchiyama, S. Intracellular Temperature Mapping with a Fluorescent Polymeric Thermometer and Fluorescence Lifetime Imaging Microscopy. Nat. Commun. 2012, 3, 705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa de Almeida, M.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding Nanoparticle Endocytosis to Improve Targeting Strategies in Nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Fukuda, N.; Uchiyama, S.; Inada, N. A Cell-Permeable Fluorescent Polymeric Thermometer for Intracellular Temperature Mapping in Mammalian Cell Lines. PLoS ONE 2015, 10, e0117677. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Matsuoka, I. Calorimetric Studies of Heat of Respiration of Mitochondria. J. Biochem. 1978, 84, 39–46. [Google Scholar] [CrossRef]
- Gota, C.; Okabe, K.; Funatsu, T.; Harada, Y.; Uchiyama, S. Hydrophilic Fluorescent Nanogel Thermometer for Intracellular Thermometry. J. Am. Chem. Soc. 2009, 131, 2766–2767. [Google Scholar] [CrossRef]
- Tsuji, T.; Ikado, K.; Koizumi, H.; Uchiyama, S.; Kajimoto, K. Difference in Intracellular Temperature Rise between Matured and Precursor Brown Adipocytes in Response to Uncoupler and β-Adrenergic Agonist Stimuli. Sci. Rep. 2017, 7, 12889. [Google Scholar] [CrossRef] [Green Version]
- Kiyonaka, S.; Kajimoto, T.; Sakaguchi, R.; Shinmi, D.; Omatsu-Kanbe, M.; Matsuura, H.; Imamura, H.; Yoshizaki, T.; Hamachi, I.; Morii, T.; et al. Genetically Encoded Fluorescent Thermosensors Visualize Subcellular Thermoregulation in Living Cells. Nat. Methods 2013, 10, 1232–1238. [Google Scholar] [CrossRef]
- Constant-Urban, C.; Charif, M.; Goffin, E.; van Heugen, J.-C.; Elmoualij, B.; Chiap, P.; Mouithys-Mickalad, A.; Serteyn, D.; Lebrun, P.; Pirotte, B.; et al. Triphenylphosphonium Salts of 1,2,4-Benzothiadiazine 1,1-Dioxides Related to Diazoxide Targeting Mitochondrial ATP-Sensitive Potassium Channels. Bioorg. Med. Chem. Lett. 2013, 23, 5878–5881. [Google Scholar] [CrossRef]
- Peng, M.; Wang, X.-Q.; Zhang, Y.; Li, C.-X.; Zhang, M.; Cheng, H.; Zhang, X.-Z. Mitochondria-Targeting Thermosensitive Initiator with Enhanced Anticancer Efficiency. ACS Appl. Bio Mater. 2019, 2, 4656–4666. [Google Scholar] [CrossRef]
- Chrétien, D.; Bénit, P.; Ha, H.-H.; Keipert, S.; El-Khoury, R.; Chang, Y.-T.; Jastroch, M.; Jacobs, H.T.; Rustin, P.; Rak, M. Mitochondria Are Physiologically Maintained at Close to 50 °C. PLoS Biol. 2018, 16, e2003992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satapathy, R.; Padhy, H.; Wu, Y.-H.; Lin, H.-C. Synthesis and Characterization of Reversible Chemosensory Polymers: Modulation of Sensitivity through the Attachment of Novel Imidazole Pendants. Chem. Eur. J. 2012, 18, 16061–16072. [Google Scholar] [CrossRef] [PubMed]
- Gomez Torres, S.; Pantenburg, I.; Meyer, G. Direct Oxidation of Europium Metal with Acetic Acid: Anhydrous Europium(III) Acetate, Eu(OAc)3, Its Sesqui-Hydrate, Eu(OAc)3(H2O)1.5, and the “Hydrogendiacetate”, [Eu(H(OAc)2)3](H2O). Z. Anorg. Allg. Chem. 2006, 632, 1989–1994. [Google Scholar] [CrossRef]
- De Silva, C.R.; Maeyer, J.R.; Wang, R.; Nichol, G.S.; Zheng, Z. Adducts of Europium β-Diketonates with Nitrogen p,P′-Disubstituted Bipyridine and Phenanthroline Ligands: Synthesis, Structural Characterization, and Luminescence Studies. Inorg. Chim. Acta 2007, 360, 3543–3552. [Google Scholar] [CrossRef]
- Sodano, F.; Rolando, B.; Spyrakis, F.; Failla, M.; Lazzarato, L.; Gazzano, E.; Riganti, C.; Fruttero, R.; Gasco, A.; Sortino, S. Tuning the Hydrophobicity of a Mitochondria-Targeted NO Photodonor. ChemMedChem 2018, 13, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Tauro, J.R.; Gemeinhart, R.A. Development of Amine-Containing Polymeric Particles. J. Biomater. Sci. Polym. Ed. 2005, 16, 1233–1244. [Google Scholar] [CrossRef] [Green Version]
- Brouwer, A.M. Standards for Photoluminescence Quantum Yield Measurements in Solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef] [Green Version]
- Resch-Genger, U.; Rurack, K. Determination of the Photoluminescence Quantum Yield of Dilute Dye Solutions (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 2005–2013. [Google Scholar] [CrossRef]
- Melnikov, A.S.; Serdobintsev, P.Y.; Vedyaykin, A.D.; Khodorkovskii, M.A. Two-Photon Absorption Cross Section for Coumarins 102, 153 and 307. J. Phys. Conf. Ser. 2017, 917, 062029. [Google Scholar] [CrossRef]
- Porrès, L.; Holland, A.; Pålsson, L.-O.; Monkman, A.P.; Kemp, C.; Beeby, A. Absolute Measurements of Photoluminescence Quantum Yields of Solutions Using an Integrating Sphere. J. Fluoresc. 2006, 16, 267–273. [Google Scholar] [CrossRef]









| Sample | λabs, nm | λexc, nm | Φ, % 3 | τ20 °C/τ40 °C, μs | Sr, %/K | σTPE, GM |
|---|---|---|---|---|---|---|
| Eu1 1 | 275, 383 | 384 | 18.3 ± 1.4 | 1016/857 | 0.9 | 133 |
| Eu2 1 | 344, 391 | 340, 407 | 25.2 ± 1.4 | 243/176 | 1.9 | 87 |
| Eu3 1 | 274, 368 | 341, 360 | 31.6 ± 2.7 | 622/463 | 1.7 | 44 |
| NPs_Eu1 2 | - | 374 | 10.2 ± 0.1 | 1017/877 | 0.8 | - |
| NPs_Eu2 2 | - | 338, 405 | 16.3 ± 0.1 | 538/430 | 1.3 | - |
| NPs_Eu2_TPP 2 | - | 292, 348 | 16.8 ± 0.1 | 555/478 | 0.8 | - |
| NPs_Eu3 2 | - | 340, 358 | 17.0 ± 0.1 | 685/604 | 0.7 | - |
| Sample | Size, nm | ζ-Potential, mV | NH2-Groups, µmol/m2 | Number of Eu Complex per Particles | Number of TPP per Particles | |||
|---|---|---|---|---|---|---|---|---|
| DLS | PDI | TEM 1 | TEM 2 | |||||
| NPs_Eu1 | 116 | 0.07 | 85 | 76 | 40.0 | 0.74 | 350 | - 3 |
| NPs_Eu2 | 100 | 0.06 | 76 | 67 | 31.6 | 0.84 | 800 | - 3 |
| NPs_Eu2_TPP | 99 | 0.11 | 76 | 67 | 24.0 | 0.07 | 800 | 22,800 |
| NPs_Eu3 | 101 | 0.14 | 79 | 69 | 22.4 | 0.82 | 425 | - 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsov, K.M.; Baigildin, V.A.; Solomatina, A.I.; Galenko, E.E.; Khlebnikov, A.F.; Sokolov, V.V.; Tunik, S.P.; Shakirova, J.R. Polymeric Nanoparticles with Embedded Eu(III) Complexes as Molecular Probes for Temperature Sensing. Molecules 2022, 27, 8813. https://doi.org/10.3390/molecules27248813
Kuznetsov KM, Baigildin VA, Solomatina AI, Galenko EE, Khlebnikov AF, Sokolov VV, Tunik SP, Shakirova JR. Polymeric Nanoparticles with Embedded Eu(III) Complexes as Molecular Probes for Temperature Sensing. Molecules. 2022; 27(24):8813. https://doi.org/10.3390/molecules27248813
Chicago/Turabian StyleKuznetsov, Kirill M., Vadim A. Baigildin, Anastasia I. Solomatina, Ekaterina E. Galenko, Alexander F. Khlebnikov, Victor V. Sokolov, Sergey P. Tunik, and Julia R. Shakirova. 2022. "Polymeric Nanoparticles with Embedded Eu(III) Complexes as Molecular Probes for Temperature Sensing" Molecules 27, no. 24: 8813. https://doi.org/10.3390/molecules27248813
APA StyleKuznetsov, K. M., Baigildin, V. A., Solomatina, A. I., Galenko, E. E., Khlebnikov, A. F., Sokolov, V. V., Tunik, S. P., & Shakirova, J. R. (2022). Polymeric Nanoparticles with Embedded Eu(III) Complexes as Molecular Probes for Temperature Sensing. Molecules, 27(24), 8813. https://doi.org/10.3390/molecules27248813