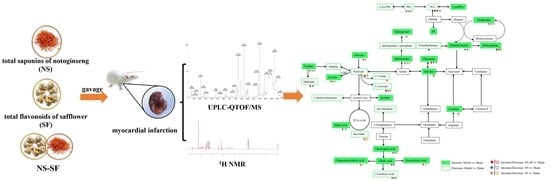
The Synergistic Mechanism of Total Saponins and Flavonoids in Notoginseng–Safflower against Myocardial Infarction Using a Comprehensive Metabolomics Strategy
Abstract
:1. Introduction
2. Results
2.1. Comparison of the Therapeutic Effects of NS, SF, and CNS in the MI Model
2.2. NS, SF, and CNS Inhibited Inflammatory Injury and Oxidative Stress Induced by MI
2.3. NS, SF, and CNS Restored Global Metabolite Abnormalities in MI Rats
2.4. Identification of Metabolic Alterations and Pathways Related to MI
2.5. NS, SF, and NS–SF Showed Different Characteristics in Improving the Differential Metabolites Related to MI
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Animals
4.3. MI Model and Drug Administration
4.4. Echocardiographic Evaluation
4.5. Sample Collection
4.6. Histological Examination
4.7. Biochemical Indicators
4.8. Sample Preparation for UPLC-QTOF/MS Analysis
4.9. Sample Preparation for NMR Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Saleh, M.; Ambrose, J.A. Understanding myocardial infarction. F1000Research 2018, 7, 1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, Z.W.; Thanikachalam, P.V.; Ramamurthy, S. Molecular understanding of the protective role of natural products on isoproterenol-induced myocardial infarction: A review. Biomed. Pharmacother. 2017, 94, 1145–1166. [Google Scholar] [CrossRef] [PubMed]
- Smit, M.; Coetzee, A.R.; Lochner, A. The pathophysiology of myocardial ischemia and perioperative myocardial infarction. J. Cardiothorac. Vasc. Anesth. 2020, 34, 2501–2512. [Google Scholar] [CrossRef]
- Femia, G.; French, J.K.; Juergens, C.; Leung, D.; Lo, S. Right ventricular myocardial infarction: Pathophysiology, clinical implications and management. Rev. Cardiovasc. Med. 2021, 22, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Layne, K.; Ferro, A. Traditional Chinese medicines in the management of cardiovascular diseases: A comprehensive systematic review. Br. J. Clin. Pharmacol. 2017, 83, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.X.; Song, Y.; Xu, W.L.; Zhang, L.X.; Li, C.; Li, Y.L. Natural herbal medicine as a treatment strategy for myocardial infarction through the regulation of angiogenesis. Evid.-Based Complement. Altern. Med. 2022, 2022, 8831750. [Google Scholar] [CrossRef]
- Meng, Y.; Du, Z.; Li, Y.; Wang, L.; Gao, P.; Gao, X.; Li, C.; Zhao, M.; Jiang, Y.; Tu, P.; et al. Integration of metabolomics with pharmacodynamics to elucidate the anti-myocardial ischemia effects of combination of notoginseng total saponins and safflower total flavonoids. Front. Pharmacol. 2018, 9, 667. [Google Scholar] [CrossRef] [Green Version]
- Rinschen, M.M.; Ivanisevic, J.; Giera, M.; Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat Rev. Mol. Cell Biol. 2019, 20, 353–367. [Google Scholar] [CrossRef]
- Long, Y.; Yang, Q.; Xiang, Y.; Zhang, Y.; Wan, J.; Liu, S.; Li, N.; Peng, W. Nose to brain drug delivery—A promising strategy for active components from herbal medicine for treating cerebral ischemia reperfusion. Pharmacol. Res. 2020, 159, 104795. [Google Scholar] [CrossRef]
- Wu, G.S.; Li, H.K.; Zhang, W.D. Metabolomics and its application in the treatment of coronary heart disease with traditional Chinese medicine. Chin. J. Cardiovasc. Med. 2019, 17, 321–330. [Google Scholar] [CrossRef]
- Liu, X.; Wei, F.; Liu, H.; Zhao, S.; Du, G.; Qin, X. Integrating hippocampal metabolomics and network pharmacology deciphers the antidepressant mechanisms of Xiaoyaosan. J. Ethnopharmacol. 2021, 268, 113549. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Y.; Li, H.X.; Ma, X.; Zhang, K.; Ma, Z.Z.; Jiang, Y.; Tu, P.F. Evaluation of the anti-myocardial ischemia effect of individual and combined extracts of Panax notoginseng and Carthamus tinctorius in rats. J. Ethnopharmacol. 2013, 145, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Huo, Y.; An, X.; Singh, G.; Chen, M.; Yang, Z.; Pu, J.; Li, J. Panax notoginseng and its components decreased hypertension via stimulation of endothelial-dependent vessel dilatation. Vasc. Pharmacol. 2012, 56, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Fu, F.H.; Han, B.; Li, G.S.; Zhang, L.M.; Liu, K. Hydroxysafflor yellow A reduces myocardial infarction size after coronary artery ligation in rats. Pharm. Biol. 2009, 47, 458–462. [Google Scholar] [CrossRef]
- Wang, C.; Ma, H.; Zhang, S.; Wang, Y.; Liu, J.; Xiao, X. Safflor yellow B suppresses pheochromocytoma cell (PC12) injury induced by oxidative stress via antioxidant system and Bcl-2/Bax pathway. Naunyn Schmiedebergs Arch. Pharmacol. 2009, 380, 135–142. [Google Scholar] [CrossRef]
- Luczak, E.D.; Wu, Y.; Granger, J.M.; Joiner, M.A.; Wilson, N.R.; Gupta, A.; Umapathi, P.; Murphy, K.R.; Reyes Gaido, O.E.; Sabet, A.; et al. Mitochondrial CaMKII causes adverse metabolic reprogramming and dilated cardiomyopathy. Nat. Commun. 2020, 11, 4416. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Caggiano, M.; Eaton, P. Heart failure-emerging roles for the mitochondrial pyruvate carrier. Cell Death Differ. 2021, 28, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Bertero, E.; Maack, C. Metabolic remodeling in heart failure. Nat. Rev. Cardiol. 2018, 15, 457–470. [Google Scholar] [CrossRef]
- Bäckström, T.; Goiny, M.; Lockowandt, U.; Liska, J.; Franco-Cereceda, A. Cardiac outflow of amino acids and purines during myocardial ischemia and reperfusion. J. Appl. Physiol. 2003, 94, 1122–1128. [Google Scholar] [CrossRef] [Green Version]
- Ameta, K.; Gupta, A.; Ameta, D.; Sethi, R.; Kumar, D.; Ahmad, I.; Mahdi, A.A. 1H NMR-derived metabolomics of filtered serum of myocardial ischemia in unstable angina patients. Clin. Chim. Acta. 2016, 456, 56–62. [Google Scholar] [CrossRef]
- Funk, A.M.; Wen, X.; Hever, T.; Maptue, N.R.; Khemtong, C.; Sherry, A.D.; Malloy, C.R. Effects of deuteration on transamination and oxidation of hyperpolarized C-pyruvate in the isolated heart. J. Magn. Reson. 2019, 301, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.S.; Azzolini, M.; Lira Ruas, J. The kynurenine connection: How exercise shifts muscle tryptophan metabolism and affects energy homeostasis, the immune system, and the brain. Am. J. Physiol. Cell Physiol. 2020, 318, C818–C830. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorgdrager, F.J.H.; Naude, P.J.W.; Kema, I.P.; Nollen, E.A.; Deyn, P.P. Tryptophan metabolism in inflammaging: From biomarker to therapeutic target. Front. Immunol. 2019, 10, 2565. [Google Scholar] [CrossRef]
- Dos Santos, L.M.; da Silva, T.M.; Azambuja, J.H.; Ramos, P.T.; Oliveira, P.S.; da Silveira, E.F.; Pedra, N.S.; Galdino, K.; do Couto, C.A.; Soares, M.S.; et al. Methionine and methionine sulfoxide treatment induces M1/classical macrophage polarization and modulates oxidative stress and purinergic signaling parameters. Mol. Cell. Biochem. 2017, 424, 69–78. [Google Scholar] [CrossRef]
- Sun, H.; Olson, K.C.; Gao, C.; Prosdocimo, D.A.; Zhou, M.; Wang, Z.; Jeyaraj, D.; Youn, J.Y.; Ren, S.; Liu, Y.; et al. Catabolic Defect of Branched-Chain Amino Acids Promotes Heart Failure. Circulation 2016, 133, 2038–2049. [Google Scholar] [CrossRef]
- Lopaschuk, G.D. Metabolic Modulators in Heart Disease: Past, Present, and Future. Can. J. Cardiol. 2017, 33, 838–849. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, S.H.; Shin, M.J.; Hwang, G.S. Alteration in metabolic signature and lipid metabolism in patients with angina pectoris and myocardial infarction. PLoS ONE 2015, 10, e0135228. [Google Scholar] [CrossRef] [Green Version]
- Bai, S.; Xie, J.; Bai, H.; Tian, T.; Zou, T.; Chen, J.J. Gut microbiota-derived inflammation-related serum metabolites as potential biomarkers for major depressive disorder. J. Inflamm. Res. 2021, 14, 3755–3766. [Google Scholar] [CrossRef]
- Cheng, M.L.; Wang, C.H.; Shiao, M.S.; Liu, M.H.; Huang, Y.Y.; Huang, C.Y.; Mao, C.T.; Lin, J.F.; Ho, H.Y.; Yang, N.I. Metabolic disturbances identified in plasma are associated with outcomes in patients with heart failure: Diagnostic and prognostic value of metabolomics. J. Am. Coll. Cardiol. 2015, 65, 1509–1520. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Q.; Wang, D.; Kong, J.; Dai, W.; Wang, X.X.; Yu, X. Common lipid features of lethal ventricular tarchyarrhythmias (LVTAs) induced by myocardial infarction and myocardial ion channel diseases. Sci. Rep. 2017, 7, 4220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Zhang, Y.; Wu, Q.; Xie, D.Z.; Dai, W.T.; Zhang, X.J.; Yang, Z.; Wang, D. Integrative analyses of myocardial lipidome and proteome implicate mitochondrial dysfunction in lethal ventricular tachyarrhythmia (LVTA) induced by acute myocardial ischemia (AMI). J. Proteom. 2019, 197, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, C.; More, M.; Bellamine, A. Trimethylamine N-Oxide in relation to cardiometabolic health-cause or effect? Nutrients 2020, 12, 1330. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Kidd, J.; Gehr, T.; Li, P.L. Podocyte sphingolipid signaling in nephrotic syndrome. Cell. Physiol. Biochem. 2021, 55, 13–34. [Google Scholar] [CrossRef]
- Quinville, B.M.; Deschenes, N.M.; Ryckman, A.E.; Walia, J.S. A Comprehensive review: Sphingolipid metabolism and implications of disruption in sphingolipid homeostasis. Int. J. Mol. Sci. 2021, 22, 5793. [Google Scholar] [CrossRef]
- Knapp, M. Cardioprotective role of sphingosine-1-phosphate. J. Physiol. Pharmacol. 2011, 62, 601–607. [Google Scholar]
- Bernacchioni, C.; Ghini, V.; Squecco, R.; Idrizaj, E.; Garella, R.; Puliti, E.; Cencetti, F.; Bruni, P.; Donati, C. Role of sphingosine 1-phosphate signaling axis in muscle atrophy induced by TNFα in C2C12 myotubes. Int. J. Mol. Sci. 2021, 22, 1280. [Google Scholar] [CrossRef]
- Li, T.; Chiang, J.Y. Bile acid signaling in metabolic disease and drug therapy. Pharmacol. Rev. 2014, 66, 948–983. [Google Scholar] [CrossRef] [Green Version]
- Chiang, J.; Ferrell, J.M. Bile acid metabolism in liver pathobiology. Gene Expr. 2018, 18, 71–87. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.Y.; Li, Y.Y.; Jadhav, K.; Pan, X.L.; Zhu, Y.D.; Hu, S.W.; Chen, S.R.; Chen, L.Y.; Tang, Y.; Wang, H.H.; et al. Hepatocyte ATF3 protects against atherosclerosis by regulating HDL and bile acid metabolism. Nat. Metab. 2021, 3, 59–74. [Google Scholar] [CrossRef]
- Du, Z.Y.; Shu, Z.L.; Lei, W.; Li, C.; Zeng, K.W.; Guo, X.; Zhao, M.B.; Tu, P.F.; Jiang, Y. Integration of metabonomics and transcriptomics reveals the therapeutic effects and mechanisms of Baoyuan Decoction for myocardial ischemia. Front. Pharmacol. 2018, 9, 514. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.H.; Huang, Y.T.; Ni, J.Y.; Chen, J.R.; Wei, J.; Gao, H.; Li, L.; Zhou, Z.C.; Wang, Y.L.; Xu, Y.S.; et al. Qi Dan Li Xin pill improves chronic heart failure by regulating mTOR/p70S6k-mediated autophagy and inhibiting apoptosis. Sci. Rep. 2020, 10, 6105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Y.Q.; Du, Z.Y.; Li, Y.; Gao, P.; Song, J.Y.; Lu, Y.Y.; Tu, P.F.; Jiang, Y.; Guo, X.Y. The synergistic mechanism of total saponins and flavonoids in Notoginseng−Safflower pair against myocardial ischemia uncovered by an integrated metabolomics strategy. Biomed. Pharmacother. 2020, 130, 110574. [Google Scholar] [CrossRef] [PubMed]
- Feriani, A.; Khdhiri, E.; Tir, M.; Elmufti, A.; Tlili, N.; Hajji, R.; Ammar, H.; Allouche, N.; Abid, S.; Ghazouani, L.; et al. (E)-N′-(1-(7-hydroxy-2-oxo-2H-chromen-3-Yl) ethylidene) benzohydrazide, a novel synthesized coumarin, ameliorates isoproterenol-induced myocardial infarction in rats through attenuating oxidative stress, inflammation, and apoptosis. Oxidative Med. Cell. Longev. 2020, 2020, 2432918. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Liu, X.; Zhang, T.; Chen, C.; Dong, X.; Can, Y.; Liu, P. Behavioral and biochemical effects of KXS on postmyocardial infarction depression. Front. Pharmacol. 2010, 11, 561817. [Google Scholar] [CrossRef] [PubMed]






| Detected | ||||||||
|---|---|---|---|---|---|---|---|---|
| No. | tR | m/z | Formula | Mode | Identification | HMDB IDs | Trend | Pathway |
| 1 | 0.75 | 299.2401 | C10H13N5O6 | – | 8-Hydroxyguanosine | HMDB0002044 | ↑ | Metabolism of nucleotides |
| 2 | 0.83 | 192.1235 | C6H8O7 | – | Citrate | HMDB0000094 | ↓ | TCA cycle |
| 3 | 5.41 | 406.5555 | C24H38O5 | – | 3-oxocholic acid | HMDB0000502 | ↑ | Bile acid metabolism |
| 4 | 5.42 | 408.5714 | C24H40O5 | – | Ursocholic acid | HMDB0000917 | ↓ | Secondary bile acid biosynthesis |
| 5 | 5.42 | 408.2875 | C24H40O5 | – | Cholic acid | HMDB0000619 | ↓ | Primary bile acid biosynthesis |
| 6 | 7.42 | 392.2926 | C24H40O4 | – | Deoxycholic acid | HMDB0000626 | ↓ | Secondary bile acid biosynthesis |
| 7 | 10.01 | 155.9823 | C2H5O6P | – | Phosphoglycolic acid | HMDB0000816 | ↑ | Glyoxylate and dicarboxylatemetabolism |
| 8 | 10.99 | 118.0266 | C4H6O4 | – | Succinate | HMDB0000254 | ↓ | TCA cycle |
| 9 | 11.66 | 292.2038 | C18H28O3 | – | alpha-Licanic acid | LMFA02000194 | ↓ | Fatty acid metabolism |
| 10 | 14.02 | 134.0874 | C4H6O5 | – | Malic acid | HMDB0000744 | ↑ | TCA cycle |
| 11 | 0.57 | 233.2616 | C10H19NO5 | + | Hydroxypropionylcarnitine | HMDB0013125 | ↑ | Fat metabolism |
| 12 | 0.64 | 202.2906 | C11H22O3 | + | 3-hydroxyundecanoic acid | HMDB0061654 | ↑ | Fatty acid metabolism |
| 13 | 1.62 | 276.2863 | C11H20N2O6 | + | Saccharopine | HMDB0000279 | ↓ | Lysine degradation |
| 14 | 2.67 | 650.2801 | C25H47O12P | + | PI (16:1/0:0) | LMGF06050009 | ↑ | Glycerophospholipid metabolism |
| 15 | 6.21 | 273.2744 | C17H31D3O2 | + | Margaric acid | LMFA01010048 | ↓ | Fatty acid metabolism |
| 16 | 6.84 | 167.1255 | C5H5N5O2 | + | 8-hydroxyguanine | HMDB0002032 | ↑ | Purines and purine derivatives |
| 17 | 7.61 | 541.3168 | C28H48NO7P | + | LysoPC (20:5) | HMDB0010397 | ↑ | Glycerophospholipid metabolism |
| 18 | 8.32 | 301.5078 | C18H39NO2 | + | Sphinganine | HMDB0000269 | ↑ | Sphingolipid metabolism |
| 19 | 8.53 | 379.4718 | C18H38NO5P | + | Sphigosine-1-phosphate | HMDB0000277 | ↓ | Sphingolipid metabolism |
| 20 | 8.86 | 523.6832 | C26H54NO7P | + | LysoPC (18:0) | HMDB0010384 | ↑ | Glycerophospholipid metabolism |
| 21 | 8.87 | 299.4919 | C18H37NO2 | + | Sphingosine | HMDB0000252 | ↓ | Sphingolipid metabolism |
| 22 | 8.87 | 495.3325 | C24H50NO7P | + | PE (19:0/0:0) | LMGP02050028 | ↓ | Glycerophospholipid metabolism |
| 23 | 9.01 | 317.2202 | C16H31NO5 | + | 3-hydroxynonanoyl carnitine | HMDB0061635 | ↓ | Fat metabolism |
| 24 | 9.33 | 392.2926 | C24H40O4 | + | Chenodeoxycholic acid | HMDB0000518 | ↑ | Primary bile acid biosynthesis |
| 25 | 9.37 | 495.3325 | C24H50NO7P | + | PC (16:0/0:0) | LMGP01050018 | ↓ | Glycerophospholipid metabolism |
| 26 | 9.39 | 527.6304 | C27H46NO7P | + | LysoPE(22:5/0:0) | HMDB0011524 | ↓ | Glycerophospholipid metabolism |
| 27 | 9.52 | 521.3481 | C26H52NO7P | + | PC (18:1/0:0) | LMGP01050029 | ↓ | Glycerophospholipid metabolism |
| 28 | 9.52 | 521.3481 | C26H52NO7P | + | LysoPC (18:1) | HMDB0002815 | ↑ | Glycerophospholipid metabolism |
| 29 | 10.01 | 375.5878 | C24H41NO2 | + | Adrenoyl ethanolamide | HMDB0013626 | ↑ | Fatty acid metabolism |
| 30 | 10.21 | 244.2014 | C9H12N2O6 | + | Uridine | HMDB0000296 | ↑ | Pyrimidine metabolism |
| 31 | 10.69 | 515.2916 | C26H45NO7S | + | Taurocholic acid | HMDB0000036 | ↓ | Primary bile acid biosynthesis |
| 32 | 10.97 | 398.3396 | C24H46O4 | + | Axillarenic acid | LMFA01050418 | ↓ | Fatty acid metabolism |
| 33 | 10.99 | 509.6566 | C25H52NO7P | + | LysoPE (20:0) | HMDB0011511 | ↑ | Glycerophospholipid metabolism |
| 34 | 11.08 | 425.3505 | C25H47NO4 | + | Vaccenyl carnitine | HMDB0006351 | ↑ | Fatty acid metabolism |
| 35 | 11.69 | 523.3638 | C26H54NO7P | + | PE (21:0/0:0) | LMGP02050026 | ↑ | Glycerophospholipid metabolism |
| 36 | 11.88 | 195.1721 | C9H9NO4 | + | 3-hydroxyhippuric acid | HMDB0006116 | ↓ | Glycerophospholipid metabolism |
| 37 | 12.03 | 523.3638 | C26H54NO7P | + | PC (18:0) | LMGP01050026 | ↓ | Glycerophospholipid metabolism |
| 38 | 12.03 | 273.1212 | C12H19NO6 | + | Glutaconylcarnitine | HMDB0013129 | ↓ | Fat metabolism |
| 39 | 12.78 | 120.1039 | C4H8O4 | + | 4-deoxyerythronic acid | HMDB0000498 | ↑ | Fatty acid metabolism |
| No. | Potential Biomarkers | 1H NMR |
|---|---|---|
| 1 | Leucine | δ 0.92 (d) |
| 2 | Valine | δ 1.04 (d), 3.61 (d) |
| 3 | Lactate | δ 1.35 (d) |
| 4 | Threonine | δ 1.36 (d), 3.58 (d) |
| 5 | Alanine | δ 1.48 (d) |
| 6 | Acetate | δ 1.94 (s) |
| 7 | Methionine | δ 2.16 (s) |
| 8 | Pyruvate | δ 2.34 (s) |
| 9 | Succinate | δ 2.40 (s) |
| 10 | 3-Hydroxybutyrate | δ 1.20 (d) |
| 11 | Methylamine | δ 2.61 (s) |
| 12 | Dimethylamine | 2.72 (s) |
| 13 | Trimethylamine | δ 2.96 (s) |
| 14 | Creatine | δ 3.04 (s) |
| 15 | Malonate | δ 3.12 (s) |
| 16 | β-glucose | δ 3.24 (d) |
| 17 | Glycine | δ 3.52 (s) |
| No. | Identification | HMDB IDs | Normal vs. Model | Positive vs. Model | CNS vs. Model | NS vs. Model | SF vs. Model |
|---|---|---|---|---|---|---|---|
| 1 | 8-Hydroxyguanosine | HMDB0002044 | Δ * | Δ * | / | Δ * | / |
| 2 | Citrate | HMDB0000094 | Δ ** | Δ ** | Δ ** | / | Δ ** |
| 3 | 3-Oxocholic acid | HMDB0000502 | Δ ** | Δ ** | Δ ** | Δ ** | Δ ** |
| 4 | Ursocholic acid | HMDB0000917 | Δ ** | / | Δ ** | Δ * | Δ * |
| 5 | Cholic acid | HMDB0000619 | Δ ** | Δ * | Δ * | Δ * | / |
| 6 | Deoxycholic acid | HMDB0000626 | Δ ** | Δ ** | Δ * | / | Δ * |
| 7 | Phosphoglycolic acid | HMDB0000816 | Δ * | / | / | / | Δ * |
| 8 | Succinate | HMDB0000254 | Δ ** | Δ ** | Δ ** | / | Δ ** |
| 9 | alpha-Licanic acid | LMFA02000194 | Δ * | Δ * | Δ * | Δ * | / |
| 10 | Malic acid | HMDB0000744 | Δ ** | / | Δ ** | Δ ** | / |
| 11 | Hydroxypropionylcarnitine | HMDB0013125 | Δ ** | / | Δ * | / | Δ * |
| 12 | 3-Hydroxyundecanoic acid | HMDB0061654 | Δ * | / | / | / | Δ * |
| 13 | Saccharopine | HMDB0000279 | Δ * | / | / | / | / |
| 14 | PI (16:1/0:0) | LMGP06050009 | Δ * | Δ * | / | / | / |
| 15 | Margaric acid | LMFA01010048 | Δ ** | Δ ** | Δ ** | Δ ** | Δ ** |
| 16 | 8-Hydroxyguanine | HMDB0002032 | Δ ** | Δ ** | Δ ** | Δ ** | / |
| 17 | LysoPC (20:5) | HMDB0010397 | Δ * | / | / | / | / |
| 18 | Sphinganine | HMDB0000269 | Δ ** | Δ ** | Δ ** | Δ * | Δ * |
| 19 | Sphingosine 1-phosphate | HMDB0000277 | Δ ** | Δ ** | Δ ** | / | / |
| 20 | LysoPC (18:0) | HMDB0010384 | Δ ** | Δ ** | Δ ** | / | / |
| 21 | Sphingosine | HMDB0000252 | Δ ** | Δ ** | Δ ** | / | / |
| 22 | PE (19:0/0:0) | LMGP02050028 | Δ ** | / | Δ ** | Δ ** | Δ ** |
| 23 | 3-Hydroxynonanoyl carnitine | HMDB0061635 | Δ * | / | / | / | / |
| 24 | Chenodeoxycholic acid | HMDB0000518 | Δ ** | Δ ** | Δ ** | / | / |
| 25 | PC (16:0/0:0) | LMGP01050018 | Δ ** | Δ ** | Δ ** | Δ ** | Δ ** |
| 26 | LysoPE (22:5/0:0) | HMDB0011524 | Δ * | / | Δ * | / | / |
| 27 | PC (18:1/0:0) | LMGP01050029 | Δ * | Δ * | / | / | / |
| 28 | LysoPC (18:1) | HMDB0002815 | Δ * | Δ * | / | Δ * | / |
| 29 | Adrenoyl ethanolamide | HMDB0013626 | Δ * | / | / | / | / |
| 30 | Uridine | HMDB0000296 | Δ ** | Δ ** | Δ ** | Δ ** | Δ * |
| 31 | Taurocholic acid | HMDB0000036 | Δ ** | Δ * | Δ ** | Δ * | Δ ** |
| 32 | Axillarenic acid | LMFA01050418 | Δ ** | Δ ** | Δ ** | Δ ** | / |
| 33 | LysoPE (20:0) | HMDB0011511 | Δ * | Δ * | / | / | / |
| 34 | Vaccenyl carnitine | HMDB0006351 | Δ ** | / | Δ ** | Δ ** | / |
| 35 | PE (21:0/0:0) | LMGP02050026 | Δ ** | Δ ** | Δ ** | Δ ** | Δ ** |
| 36 | 3-Hydroxyhippuric acid | HMDB0006116 | Δ ** | / | Δ * | / | / |
| 37 | PC (18:0) | LMGP01050026 | Δ ** | Δ ** | Δ ** | Δ ** | Δ ** |
| 38 | Glutaconylcarnitine | HMDB0013129 | Δ * | Δ * | Δ * | / | Δ * |
| 39 | 4-Deoxyerythronic acid | HMDB0000498 | Δ ** | / | Δ * | / | Δ * |
| 40 | L-Leucine | HMDB0000687 | Δ ** | Δ ** | Δ ** | Δ * | Δ ** |
| 41 | L-Valine | HMDB0000883 | Δ ** | / | Δ ** | / | / |
| 42 | Lactate | HMDB0000190 | Δ ** | Δ ** | Δ ** | Δ * | Δ * |
| 43 | Threonine | HMDB0000167 | Δ ** | Δ ** | Δ ** | Δ ** | Δ * |
| 44 | Alanine | HMDB0000161 | Δ * | / | / | / | / |
| 45 | Acetate | HMDB0000042 | Δ * | / | / | / | / |
| 46 | Methionine | HMDB0000696 | Δ * | / | / | / | / |
| 47 | Pyruvate | HMDB0000243 | Δ ** | Δ ** | Δ ** | / | Δ * |
| 48 | Succinate | HMDB0000254 | Δ ** | Δ ** | Δ ** | / | Δ * |
| 49 | 3-Hydroxybutyrate | HMDB0000357 | Δ * | / | / | / | / |
| 50 | Methylamine | HMDB0000164 | Δ ** | Δ ** | Δ ** | Δ ** | Δ ** |
| 51 | Dimethylamine | HMDB0000087 | Δ ** | Δ ** | Δ ** | Δ ** | / |
| 52 | Trimethylamine | HMDB0000906 | Δ ** | Δ ** | / | Δ ** | / |
| 53 | Creatinem | HMDB0000064 | Δ ** | / | Δ ** | / | / |
| 54 | Malonate | HMDB0000691 | Δ ** | Δ ** | Δ ** | Δ * | / |
| 55 | β-Glucose | HMDB0000122 | Δ ** | Δ ** | Δ ** | / | Δ * |
| 56 | Glycine | HMDB0000123 | Δ ** | Δ ** | Δ ** | / | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, M.; Meng, Y.; Du, Z.; Guo, M.; Jiang, Y.; Tu, P.; Hua, K.; Lu, Y.; Guo, X. The Synergistic Mechanism of Total Saponins and Flavonoids in Notoginseng–Safflower against Myocardial Infarction Using a Comprehensive Metabolomics Strategy. Molecules 2022, 27, 8860. https://doi.org/10.3390/molecules27248860
Fang M, Meng Y, Du Z, Guo M, Jiang Y, Tu P, Hua K, Lu Y, Guo X. The Synergistic Mechanism of Total Saponins and Flavonoids in Notoginseng–Safflower against Myocardial Infarction Using a Comprehensive Metabolomics Strategy. Molecules. 2022; 27(24):8860. https://doi.org/10.3390/molecules27248860
Chicago/Turabian StyleFang, Meng, Yuqing Meng, Zhiyong Du, Mengqiu Guo, Yong Jiang, Pengfei Tu, Kun Hua, Yingyuan Lu, and Xiaoyu Guo. 2022. "The Synergistic Mechanism of Total Saponins and Flavonoids in Notoginseng–Safflower against Myocardial Infarction Using a Comprehensive Metabolomics Strategy" Molecules 27, no. 24: 8860. https://doi.org/10.3390/molecules27248860
APA StyleFang, M., Meng, Y., Du, Z., Guo, M., Jiang, Y., Tu, P., Hua, K., Lu, Y., & Guo, X. (2022). The Synergistic Mechanism of Total Saponins and Flavonoids in Notoginseng–Safflower against Myocardial Infarction Using a Comprehensive Metabolomics Strategy. Molecules, 27(24), 8860. https://doi.org/10.3390/molecules27248860