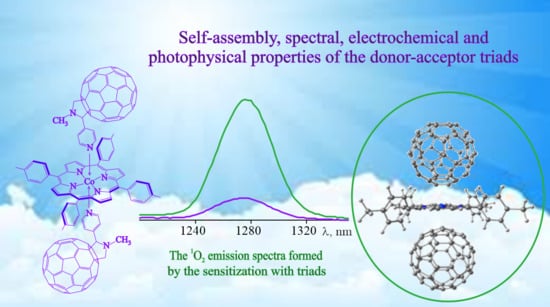
Donor–Acceptor Complexes of (5,10,15,20-Tetra(4-methylphenyl)porphyrinato)cobalt(II) with Fullerenes C60: Self-Assembly, Spectral, Electrochemical and Photophysical Properties
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis and Analysis of Compounds
3.3. Thermodynamics and Kinetics
3.4. Fluorescence Spectroscopy
3.5. Femtosecond Laser Photolysis Setup
3.6. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanzi, L.; Terreni, M.; Zhang, Y. Synthesis and biological application of glyco- and peptide derivatives of fullerene C60. Eur. J. Med. Chem. 2022, 230, 114104. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, X.; Zhang, W.; Liu, Z.; Zeng, G.; Shao, B.; Liang, Q.; He, Q.; Yuan, X.; Huang, D.; et al. Advances in photocatalysis based on fullerene C60 and its derivatives: Properties, mechanism, synthesis, and applications. Appl. Catal. B Environ. 2020, 265, 118579. [Google Scholar] [CrossRef]
- Heredia, D.A.; Durantini, A.M.; Durantini, J.E.; Durantini, E.N. Fullerene C60 derivatives as antimicrobial photodynamic agents. J. Photochem. Photobiol. C Photochem. Rev. 2022, 51, 100471. [Google Scholar] [CrossRef]
- Yan, W.; Seifermann, S.M.; Pierrat, P.; Bräse, S. Synthesis of highly functionalized C60 fullerene derivatives and their applications in material and life sciences. Org. Biomol. Chem. 2015, 13, 25–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.-N.; Li, B.; Fu, L.; Yin, L.-W. One-step-spin-coating route for homogeneous perovskite/pyrrole-C60 fullerene bulk heterojunction for high performance solar cells. J. Power Sources 2019, 419, 27–34. [Google Scholar] [CrossRef]
- Porcu, P.; Estrada-Montaño, A.S.; Vonlanthen, M.; Cuétara-Guadarrama, F.; González-Méndez, I.; Sorroza-Martínez, K.; Zaragoza-Galán, G.; Rivera, E. Azobenzene dyads containing fullerene, porphyrin and pyrene chromophores: Molecular design and optical properties. Dye. Pigment. 2022, 197, 109858. [Google Scholar] [CrossRef]
- Stasheuski, A.S.; Galievsky, V.A.; Stupak, A.P.; Dzhagarov, B.M.; Choi, M.J.; Chung, B.H.; Jeong, J.Y. Photophysical Properties and Singlet Oxygen Generation Efficiencies of Water-Soluble Fullerene Nanoparticles. Photochem. Photobiol. 2014, 90, 997–1003. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Mauter, M.S.; Elimelech, M. Microbial Cytotoxicity of Carbon-Based Nanomaterials: Implications for River Water and Wastewater Effluent. Environ. Sci. Technol. 2009, 43, 2648–2653. [Google Scholar] [CrossRef]
- Lyon, D.Y.; Brunet, L.; Hinkal, G.W.; Wiesner, M.R.; Alvarez, P.J.J. Antibacterial Activity of Fullerene Water Suspensions (nC60) Is Not Due to ROS-Mediated Damage. Nano Lett. 2008, 8, 1539–1543. [Google Scholar] [CrossRef]
- Kubatova, H.; Zemanova, E.; Klouda, K.; Bilek, K.; Kadukova, J. Effects of C60 Fullerene and its Derivatives on Selected Microorganisms. J. Microbiol. Res. 2013, 3, 152–162. [Google Scholar] [CrossRef]
- Fedorova, N.E.; Klimova, R.R.; Tulenev, Y.A.; Chichev, E.V.; Kornev, A.B.; Troshin, P.A.; Kushch, A.A. Carboxylic Fullerene C60 Derivatives: Efficient Microbicides Against Herpes Simplex Virus And Cytomegalovirus Infections In Vitro. Mendeleev Commun. 2012, 22, 254–256. [Google Scholar] [CrossRef]
- Wang, M.; Maragani, S.; Huang, L.; Jeon, S.; Canteenwala, T.; Hamblin, M.R.; Chiang, L.Y. Synthesis of decacationic [60]fullerene decaiodides giving photoinduced production of superoxide radicals and effective PDT-mediation on antimicrobial photoinactivation. Eur. J. Med. Chem. 2013, 63, 170–184. [Google Scholar] [CrossRef] [Green Version]
- Siddiquie, R.Y.; Agrawal, A.; Joshi, S.S. Surface Alterations to Impart Antiviral Properties to Combat COVID-19 Transmission. Trans. Indian Natl. Acad. Eng. 2020, 5, 343–347. [Google Scholar] [CrossRef]
- Vorobiev, A.K.; Markov, V.Y.; Samokhvalova, N.A.; Samokhvalov, P.S.; Troyanov, S.I.; Sidorov, L.N. Stable trifluoromethylated fullerene radicals C60(CF3)15 and C60(CF3)17. Mendeleev Commun. 2010, 20, 7–9. [Google Scholar] [CrossRef]
- Samokhvalova, N.A.; Khavrel’, P.A.; Goryunkov, A.A.; Ioffe, I.N.; Karnatsevich, V.L.; Sidorov, L.N.; Kemnitz, E.; Troyanova, S.I. New isomers of trifluoromethylated fullerene: C60(CF3)12 and C60(CF3)14. Russ. Chem. Bull. 2008, 57, 2526–2534. [Google Scholar] [CrossRef]
- Brotsman, V.A.; Ioutsi, V.A.; Rybalchenko, A.V.; Markov, V.Y.; Belov, N.M.; Lukonina, N.S.; Troyanov, S.I.; Ioffe, I.N.; Trukhanov, V.A.; Galimova, G.K.; et al. Tightly Bound Double-Caged [60]Fullerene Derivatives with Enhanced Solubility: Structural Features and Application in Solar Cells. Chem. Asian J. 2017, 12, 1075–1086. [Google Scholar] [CrossRef]
- Brotsman, V.A.; Rybalchenko, A.V.; Zubov, D.N.; Paraschuk, D.Y.; Goryunkov, A.A. Double-caged fullerene acceptors: Effect of alkyl chain length on photovoltaic performance. J. Mater. Chem. C 2019, 7, 3278–3285. [Google Scholar] [CrossRef]
- Harneit, W.; Meyer, C.; Weidinger, A.; Suter, D.; Twamley, J. Architectures for a Spin Quantum Computer Based on Endohedral Fullerenes. Phys. Status Solidi (b) 2002, 233, 453–461. [Google Scholar] [CrossRef]
- Troshin, P.A.; Peregudov, A.S.; Troyanov, S.I.; Lyubovskaya, R.N. New pyrrolidine and pyrroline derivatives of fullerenes: From the synthesis to the use in light-converting systems. Russ. Chem. Bull. 2008, 57, 887–912. [Google Scholar] [CrossRef]
- Kc, C.B.; D’Souza, F. Design and photochemical study of supramolecular donor–acceptor systems assembled via metal–ligand axial coordination. Coord. Chem. Rev. 2016, 322, 104–141. [Google Scholar] [CrossRef]
- Borges-Martínez, M.; Montenegro-Pohlhammer, N.; Zhang, X.; Galvez-Aranda, D.E.; Ponce, V.; Seminario, J.M.; Cárdenas-Jirón, G. Fullerene binding effects in Al(III)/Zn(II) Porphyrin/Phthalocyanine photophysical properties and charge transport. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 269, 120740. [Google Scholar] [CrossRef] [PubMed]
- Kulyk, B.; Waszkowska, K.; Busseau, A.; Villegas, C.; Hudhomme, P.; Dabos-Seignon, S.; Zawadzka, A.; Legoupy, S.; Sahraoui, B. Penta(zinc porphyrin)[60]fullerenes: Strong reverse saturable absorption for optical limiting applications. Appl. Surf. Sci. 2020, 533, 147468. [Google Scholar] [CrossRef]
- Costa, J.I.T.; Farinha, A.S.F.; Neves, M.; Tome, A.C. An easy access to porphyrin triads and their supramolecular interaction with a pyridyl 60 fulleropyrrolidine. Dye. Pigment. 2016, 135, 163–168. [Google Scholar] [CrossRef]
- Guldi, D.M. Fullerenes: Three dimensional electron acceptor materials. Chem. Commun. 2000, 321–327. [Google Scholar] [CrossRef]
- Ovchenkova, E.N.; Bichan, N.G.; Gruzdev, M.S.; Ksenofontov, A.A.; Gostev, F.E.; Shelaev, I.V.; Nadtochenko, V.A.; Lomova, T.N. Carbazole-functionalized cobalt(ii) porphyrin axially bonded with C60/C70 derivatives: Synthesis and characterization. New J. Chem. 2021, 45, 9053–9065. [Google Scholar] [CrossRef]
- Bichan, N.G.; Ovchenkova, E.N.; Ksenofontov, A.A.; Mozgova, V.A.; Gruzdev, M.S.; Chervonova, U.V.; Shelaev, I.V.; Lomova, T.N. Meso-carbazole substituted porphyrin complexes: Synthesis and spectral properties according to experiment, DFT calculations and the prediction by machine learning methods. Dye. Pigment. 2022, 204, 110470. [Google Scholar] [CrossRef]
- Morisue, M.; Saito, G.; Sasada, D.; Umeyama, T.; Imahori, H.; Mitamura, K.; Masunaga, H.; Hoshino, T.; Sakurai, S.; Sasaki, S. Glassy Porphyrin/C60 Composites: Morphological Engineering of C60 Fullerene with Liquefied Porphyrins. Langmuir 2020, 36, 13583–13590. [Google Scholar] [CrossRef]
- Pratik, S.M.; Gagliardi, L.; Cramer, C.J. Boosting Photoelectric Conductivity in Porphyrin-Based MOFs Incorporating C60. J. Phys. Chem. C 2020, 124, 1878–1887. [Google Scholar] [CrossRef]
- Olmstead, M.M.; Costa, D.A.; Maitra, K.; Noll, B.C.; Phillips, S.L.; Van Calcar, P.M.; Balch, A.L. Interaction of Curved and Flat Molecular Surfaces. The Structures of Crystalline Compounds Composed of Fullerene (C60, C60O, C70, and C120O) and Metal Octaethylporphyrin Units. J. Am. Chem. Soc. 1999, 121, 7090–7097. [Google Scholar] [CrossRef]
- Konarev, D.V.; Neretin, I.S.; Slovokhotov, Y.L.; Yudanova, E.I.; Drichko, N.V.; Shul’ga, Y.M.; Tarasov, B.P.; Gumanov, L.L.; Batsanov, A.S.; Howard, J.A.K.; et al. New molecular complexes of fullerenes C60 and C70 with tetraphenylporphyrins [M(tpp)], in which M = H2, Mn, Co, Cu, Zn, and FeCl. Chem. Eur. J. 2001, 7, 2605–2616. [Google Scholar] [CrossRef]
- Sun, D.; Tham, F.S.; Reed, C.A.; Chaker, L.; Boyd, P.D.W. Supramolecular Fullerene-Porphyrin Chemistry. Fullerene Complexation by Metalated “Jaws Porphyrin” Hosts. J. Am. Chem. Soc. 2002, 124, 6604–6612. [Google Scholar] [CrossRef] [Green Version]
- Yanagisawa, M.; Tashiro, K.; Yamasaki, M.; Aida, T. Hosting Fullerenes by Dynamic Bond Formation with an Iridium Porphyrin Cyclic Dimer: A “Chemical Friction” for Rotary Guest Motions. J. Am. Chem. Soc. 2007, 129, 11912–11913. [Google Scholar] [CrossRef]
- Yamada, M.; Murakami, G.; Kobayashi, S.; Maeda, Y. Enhancing solution-phase supramolecular interactions between monomeric porphyrins and [60]fullerene by simple chemical modification. Tetrahedron Lett. 2017, 58, 4514–4518. [Google Scholar] [CrossRef]
- Nayak, S.; Ray, A.; Bhattacharya, S. Size selective supramolecular interaction upon molecular complexation of a designed porphyrin with C60 and C70 in solution. J. Mol. Liq. 2021, 321, 114367. [Google Scholar] [CrossRef]
- Bichan, N.G.; Ovchenkova, E.N.; Mozgova, V.A.; Kudryakova, N.O.; Lomova, T.N. Formation Reaction, Spectroscopy, and Photoelectrochemistry of the Donor–Acceptor Complex (5,10,15,20-Tetraphenyl-21,23H-porphinato)cobalt(II) with Pyridyl-Substituted Fullero[60]pyrrolidine. Russ. J. Inorg. Chem. 2019, 64, 605–614. [Google Scholar] [CrossRef]
- Bichan, N.G.; Ovchenkova, E.N.; Gruzdev, M.S.; Lomova, T.N. Formation Reaction and Chemical Structure of a Novel Supramolecular Triad Based on Cobalt(II) 5,10,15,20-(Tetra-4-Tert-Butylphenyl)-21H,23H-Porphyrin and 1-Methyl-2-(Pyridin-4′-yl)-3,4-Fullero 60 Pyrrolidine. J. Struct. Chem. 2018, 59, 711–719. [Google Scholar] [CrossRef]
- Bichan, N.G.; Ovchenkova, E.N.; Mozgova, V.A.; Kudryakova, N.O.; Gruzdev, M.S.; Lomova, T.N. Mechanism of the Self-Assembly of Donor–Acceptor Triads Based on Cobalt(II) Porphyrin Complex and Fullero[60]pyrrolidine, According to Data Obtained by Spectroscopic and Electrochemical Means. Russ. J. Phys. Chem. A 2020, 94, 1159–1166. [Google Scholar] [CrossRef]
- Bichan, N.G.; Ovchenkova, E.N.; Mozgova, V.A.; Kudryakova, N.O.; Lomova, T.N. Three cobalt(II) porphyrins ligated with pyridyl-containing nanocarbon/gold(III) porphyrin for solar cells: Synthesis and characterization. Polyhedron 2021, 203, 115223. [Google Scholar] [CrossRef]
- Gacka, E.; Burdzinski, G.; Marciniak, B.; Kubas, A.; Lewandowska-Andralojc, A. Interaction of light with a non-covalent zinc porphyrin–graphene oxide nanohybrid. Phys. Chem. Chem. Phys. 2020, 22, 13456–13466. [Google Scholar] [CrossRef]
- Ovchenkova, E.N.; Bichan, N.G.; Ksenofontov, A.A.; Lomova, T.N. New dyads based on trifluoromethylated phthalocyanine derivatives and substituted fullerene with possible application photoinduced electron transfer. J. Fluor. Chem. 2019, 224, 113–120. [Google Scholar] [CrossRef]
- Poddutoori, P.K.; Lim, G.N.; Vassiliev, S.; D’Souza, F. Ultrafast charge separation and charge stabilization in axially linked ‘tetrathiafulvalene–aluminum(iii) porphyrin–gold(iii) porphyrin’ reaction center mimics. Phys. Chem. Chem. Phys. 2015, 17, 26346–26358. [Google Scholar] [CrossRef] [PubMed]
- Subedi, D.R.; Jang, Y.; Ganesan, A.; Schoellhorn, S.; Reid, R.; Verbeck, G.F.; D’Souza, F. Donor-acceptor conjugates derived from cobalt porphyrin and fullerene via metal-ligand axial coordination: Formation and excited state charge separation. J. Porphyr. Phthalocyanines 2021, 25, 533–546. [Google Scholar] [CrossRef]
- Ma, B.; Sun, Y.-P. Fluorescence spectra and quantum yields of [60]fullerene and [70]fullerene under different solvent conditions. A quantitative examination using a near-infrared-sensitive emission spectrometer. J. Chem. Soc. Perkin Trans. 1996, 2, 2157–2162. [Google Scholar] [CrossRef]
- Brites, M.J.; Santos, C.; Nascimento, S.; Gigante, B.; Luftmann, H.; Fedorov, A.; Berberan-Santos, M.N. Synthesis and fluorescence properties of [60] and [70]fullerene–coumarin dyads: Efficient dipole–dipole resonance energy transfer from coumarin to fullerene. New J. Chem. 2006, 30, 1036–1045. [Google Scholar] [CrossRef]
- Catalan, J.; Elguero, J. Fluorescence of fullerenes (C60 and C70). J. Am. Chem. Soc. 1993, 115, 9249–9252. [Google Scholar] [CrossRef]
- Song, J.; Li, F.-m.; Qian, S.-x.; Li, Y.-f.; Peng, W.-j.; Zhou, J.-y.; Yu, Z.-x. Time decay behavior of fullerene-C60 studied by time-resolved photoluminescence. Acta Phys. Sin. (Overseas Ed.) 1995, 4, 175. [Google Scholar] [CrossRef]
- Shihong, M.; Xingze, L.; Jianhua, X.; Zhigang, C.; Jianying, Z.; Wencheng, W.; Zhiming, Z. Photoluminescence of fullerene[60] in ultrathin ordered multilayers: The dissociation of molecular aggregation. Chin. Sci. Bull. 1998, 43, 1004–1008. [Google Scholar] [CrossRef]
- Prat, F.; Martí, C.; Nonell, S.; Zhang, X.; Foote, C.S.; Moreno, R.G.; Bourdelande, J.L.; Font, J. C60 Fullerene-based materials as singlet oxygen O2(1Δg) photosensitizers: A time-resolved near-IR luminescence and optoacoustic study. Phys. Chem. Chem. Phys. 2001, 3, 1638–1643. [Google Scholar] [CrossRef]
- Ooyama, Y.; Enoki, T.; Ohshita, J.; Kamimura, T.; Ozako, S.; Koide, T.; Tani, F. Singlet oxygen generation properties of an inclusion complex of cyclic free-base porphyrin dimer and fullerene C60. RSC Adv. 2017, 7, 18690–18695. [Google Scholar] [CrossRef] [Green Version]
- Hung, R.R.; Grabowski, J.J. A precise determination of the triplet energy of carbon (C60) by photoacoustic calorimetry. J. Phys. Chem. 1991, 95, 6073–6075. [Google Scholar] [CrossRef]
- Hamano, T.; Okuda, K.; Mashino, T.; Hirobe, M.; Arakane, K.; Ryu, A.; Mashiko, S.; Nagano, T. Singlet oxygen production from fullerene derivatives: Effect of sequential functionalization of the fullerene core. Chem. Commun. 1997, 21–22. [Google Scholar] [CrossRef]
- Prat, F.; Stackow, R.; Bernstein, R.; Qian, W.; Rubin, Y.; Foote, C.S. Triplet-State Properties and Singlet Oxygen Generation in a Homologous Series of Functionalized Fullerene Derivatives. J. Phys. Chem. A 1999, 103, 7230–7235. [Google Scholar] [CrossRef]
- Ovchenkova, E.N.; Klyueva, M.E.; Lomova, T.N. Pyridine coordination to manganese(III) porphyrins: The effect of multiple functional substitution in porphyrin. Russ. J. Inorg. Chem. 2017, 62, 1483–1487. [Google Scholar] [CrossRef]
- Ovchenkova, E.N.; Bichan, N.G.; Lomova, T.N. Synthesis and properties of a new (octaethylporphyrinato)-manganese(III)-pyridinyl-substituted pyrrolidinofullerene dyad. Russ. J. Org. Chem. 2016, 52, 1503–1508. [Google Scholar] [CrossRef]
- Bichan, N.G.; Ovchenkova, E.N.; Kudryakova, N.O.; Ksenofontov, A.A.; Gruzdev, M.S.; Lomova, T.N. Self-assembled cobalt(ii)porphyrin–fulleropyrrolidine triads via axial coordination with photoinduced electron transfer. New J. Chem. 2018, 42, 12449–12456. [Google Scholar] [CrossRef]
- Qu, Y.; Yu, W.; Liang, S.; Li, S.; Zhao, J.; Piao, G. Structure and Morphology Characteristics of Fullerene C60 Nanotubes Fabricated with N-Methyl-2-pyrrolidone as a Good Solvent. J. Nanomater. 2011, 2011, 706293. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.-E.; Yang, W.-J.; Zhou, Y.; Pan, W.-H.; Wei, C.-X.; Yuen, A.C.Y.; Chen, T.B.Y.; Yeoh, G.H.; Lu, H.-D.; Yang, W. Synthesis of zinc porphyrin complex for improving mechanical, UV-resistance, thermal stability and fire safety properties of polystyrene. Chem. Eng. J. 2022, 442, 136367. [Google Scholar] [CrossRef]
- Jin, J.; Dong, Z.; He, J.; Li, R.; Ma, J. Synthesis of Novel Porphyrin and its Complexes Covalently Linked to Multi-Walled Carbon Nanotubes and Study of their Spectroscopy. Nanoscale Res. Lett. 2009, 4, 578. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Ozoemena, K.I.; Maree, D.M.; Nyokong, T. Synthesis and electrochemical studies of a covalently linked cobalt(ii) phthalocyanine–cobalt(ii) porphyrin conjugate. Dalton Trans. 2005, 1241–1248. [Google Scholar] [CrossRef]
- D’Souza, F.; Deviprasad, G.R.; Zandler, M.E.; Hoang, V.T.; Klykov, A.; VanStipdonk, M.; Perera, A.; El-Khouly, M.E.; Fujitsuka, M.; Ito, O. Spectroscopic, Electrochemical, and Photochemical Studies of Self-Assembled via Axial Coordination Zinc Porphyrin−Fulleropyrrolidine Dyads. J. Phys. Chem. A 2002, 106, 3243–3252. [Google Scholar] [CrossRef]
- Troshin, P.A.; Peregudov, A.S.; Mühlbacher, D.; Lyubovskaya, R.N. An Efficient [2+3] Cycloaddition Approach to the Synthesis of Pyridyl-Appended Fullerene Ligands. Eur. J. Org. Chem. 2005, 2005, 3064–3074. [Google Scholar] [CrossRef]
- Ovchenkova, E.N.; Motorina, E.V.; Bichan, N.G.; Gostev, F.E.; Lomova, T.N. Self-assembling cobalt(II) porphyrin—Fullero [60]pyrrolidine triads. Synthesis and spectral properties in the ground and excited state. J. Organomet. Chem. 2022, 977, 122458. [Google Scholar] [CrossRef]
- Ovchenkova, E.N.; Tsaturyan, A.A.; Bichan, N.G.; Lomova, T.N. N Basicity of Substituted Fullero[60]/[70]pyrrolidines According to DFT/TD-DFT Calculations and Chemical Thermodynamics. J. Phys. Chem. A 2021, 125, 5365–5374. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, S.A.; Dobryakov, A.L.; Ruthmann, J.; Ernsting, N.P. Femtosecond spectroscopy of condensed phases with chirped supercontinuum probing. Phys. Rev. A 1999, 59, 2369–2384. [Google Scholar] [CrossRef]
- Shelaev, I.V.; Gostev, F.E.; Vishnev, M.I.; Shkuropatov, A.Y.; Ptushenko, V.V.; Mamedov, M.D.; Sarkisov, O.M.; Nadtochenko, V.A.; Semenov, A.Y.; Shuvalov, V.A. P680 (PD1PD2) and ChlD1 as alternative electron donors in photosystem II core complexes and isolated reaction centers. J. Photochem. Photobiol. B Biol. 2011, 104, 44–50. [Google Scholar] [CrossRef]
- Dobryakov, A.L.; Pérez Lustres, J.L.; Kovalenko, S.A.; Ernsting, N.P. Femtosecond transient absorption with chirped pump and supercontinuum probe: Perturbative calculation of transient spectra with general lineshape functions, and simplifications. Chem. Phys. 2008, 347, 127–138. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2019.
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Zhurko, G.A. Chemcraft- Graphical Program for Visualization of Quantum Chemistry Computations, Ver. 1.8. Available online: http://www.chemcraftprog.com (accessed on 19 November 2022).













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bichan, N.G.; Ovchenkova, E.N.; Mozgova, V.A.; Ksenofontov, A.A.; Kudryakova, N.O.; Shelaev, I.V.; Gostev, F.E.; Lomova, T.N. Donor–Acceptor Complexes of (5,10,15,20-Tetra(4-methylphenyl)porphyrinato)cobalt(II) with Fullerenes C60: Self-Assembly, Spectral, Electrochemical and Photophysical Properties. Molecules 2022, 27, 8900. https://doi.org/10.3390/molecules27248900
Bichan NG, Ovchenkova EN, Mozgova VA, Ksenofontov AA, Kudryakova NO, Shelaev IV, Gostev FE, Lomova TN. Donor–Acceptor Complexes of (5,10,15,20-Tetra(4-methylphenyl)porphyrinato)cobalt(II) with Fullerenes C60: Self-Assembly, Spectral, Electrochemical and Photophysical Properties. Molecules. 2022; 27(24):8900. https://doi.org/10.3390/molecules27248900
Chicago/Turabian StyleBichan, Nataliya G., Ekaterina N. Ovchenkova, Varvara A. Mozgova, Alexander A. Ksenofontov, Nadezhda O. Kudryakova, Ivan V. Shelaev, Fedor E. Gostev, and Tatyana N. Lomova. 2022. "Donor–Acceptor Complexes of (5,10,15,20-Tetra(4-methylphenyl)porphyrinato)cobalt(II) with Fullerenes C60: Self-Assembly, Spectral, Electrochemical and Photophysical Properties" Molecules 27, no. 24: 8900. https://doi.org/10.3390/molecules27248900
APA StyleBichan, N. G., Ovchenkova, E. N., Mozgova, V. A., Ksenofontov, A. A., Kudryakova, N. O., Shelaev, I. V., Gostev, F. E., & Lomova, T. N. (2022). Donor–Acceptor Complexes of (5,10,15,20-Tetra(4-methylphenyl)porphyrinato)cobalt(II) with Fullerenes C60: Self-Assembly, Spectral, Electrochemical and Photophysical Properties. Molecules, 27(24), 8900. https://doi.org/10.3390/molecules27248900