Cerium-Doped Iron Oxide Nanorod Arrays for Photoelectrochemical Water Splitting
Abstract
:1. Introduction
2. Results
2.1. Structure and Morphology
2.2. Chemical Composition and Surface Information
3. Discussion
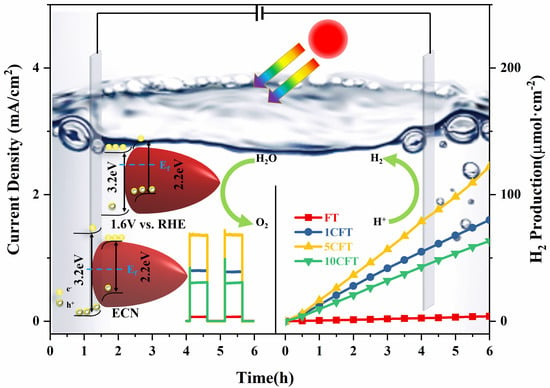
3.1. Photoelectrochemical Performance
3.2. Photoelectrochemical Mechanism for Water Splitting under Solar Light
4. Materials and Methods
4.1. Materials and Synthesis Methods
4.2. Photoelectrochemical (PEC) Testing
4.3. Materials Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Østergaard, P.A.; Duic, N.; Noorollahi, Y.; Mikulcic, H.; Kalogirou, S. Sustainable development using renewable energy technology. Renew. Energy 2020, 146, 2430–2437. [Google Scholar] [CrossRef]
- Scheffe, J.R.; Haussener, S.; Patzke, G.R. Solar Hydrogen Production. Energy Technol. 2022, 10, 2101021. [Google Scholar] [CrossRef]
- Voloshchenko, G.N. Production of Hydrogen through High-Temperature Electrolysis of Water. Nanotechnol. Russ. 2020, 15, 333–340. [Google Scholar] [CrossRef]
- Cai, L.; He, T.; Xiang, Y.; Guan, Y. Study on the reaction pathways of steam methane reforming for H2 production. Energy 2020, 207, 118296. [Google Scholar] [CrossRef]
- Ager, J.W.; Shaner, M.R.; Walczak, K.A.; Sharp, I.D.; Ardo, S. Experimental demonstrations of spontaneous, solar-driven photoelectrochemical water splitting. Energy Environ. Sci. 2015, 8, 2811–2824. [Google Scholar] [CrossRef] [Green Version]
- Centi, G.; Perathoner, S. Towards solar fuels from water and CO2. ChemSusChem 2010, 3, 195–208. [Google Scholar] [CrossRef]
- Kment, S.; Riboni, F.; Pausova, S.; Wang, L.; Wang, L.; Han, H.; Hubicka, Z.; Krysa, J.; Schmuki, P.; Zboril, R. Photoanodes based on TiO2 and alpha-Fe2O3 for solar water splitting—Superior role of 1D nanoarchitectures and of combined heterostructures. Chem. Soc. Rev. 2017, 46, 3716–3769. [Google Scholar] [CrossRef]
- Lee, J.-W.; Cho, K.-H.; Yoon, J.-S.; Kim, Y.-M.; Sung, Y.-M. Photoelectrochemical water splitting using one-dimensional nanostructures. J. Mater. Chem. A 2021, 9, 21576–21606. [Google Scholar] [CrossRef]
- Mishra, M.; Chun, D.-M. α-Fe2O3 as a photocatalytic material: A review. Appl. Catal. A Gen. 2015, 498, 126–141. [Google Scholar] [CrossRef]
- Qiu, Y.; Pan, Z.; Chen, H.; Ye, D.; Guo, L.; Fan, Z.; Yang, S. Current progress in developing metal oxide nanoarrays-based photoanodes for photoelectrochemical water splitting. Sci. Bull. 2019, 64, 1348–1380. [Google Scholar] [CrossRef]
- Ge, M.; Li, Q.; Cao, C.; Huang, J.; Li, S.; Zhang, S.; Chen, Z.; Zhang, K.; Al-Deyab, S.S.; Lai, Y. One-dimensional TiO2 Nanotube Photocatalysts for Solar Water Splitting. Adv. Sci. 2017, 4, 1600152. [Google Scholar] [CrossRef] [PubMed]
- Lianos, P. Review of recent trends in photoelectrocatalytic conversion of solar energy to electricity and hydrogen. Appl. Catal. B Environ. 2017, 210, 235–254. [Google Scholar] [CrossRef]
- Olowoyo, J.O.; Kriek, R.J. Mono- and bimetallic oxides as photo-electrocatalysts for the oxygen evolution reaction—A review. J. Phys. Chem. Solids 2022, 169, 110868. [Google Scholar] [CrossRef]
- Orimolade, B.O.; Arotiba, O.A. Bismuth vanadate in photoelectrocatalytic water treatment systems for the degradation of organics: A review on recent trends. J. Electroanal. Chem. 2020, 878, 114724. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, X.; Liu, Y.; Guan, X.; Burda, C.; Guo, L. Metal Oxide-Based Tandem Cells for Self-Biased Photoelectrochemical Water Splitting. ACS Energy Lett. 2020, 5, 844–866. [Google Scholar] [CrossRef]
- Sun, M.; Gao, R.T.; He, J.; Liu, X.; Nakajima, T.; Zhang, X.; Wang, L. Photo-driven Oxygen Vacancies Extends Charge Carrier Lifetime for Efficient Solar Water Splitting. Angew. Chem. Int. Ed. 2021, 60, 17601–17607. [Google Scholar] [CrossRef]
- Yi, S.-S.; Wulan, B.-R.; Yan, J.-M.; Jiang, Q. Highly Efficient Photoelectrochemical Water Splitting: Surface Modification of Cobalt-Phosphate-Loaded Co3O4/Fe2O3 p–n Heterojunction Nanorod Arrays. Adv. Funct. Mater. 2019, 29, 1801902. [Google Scholar] [CrossRef]
- Bak, A.; Choi, W.; Park, H. Enhancing the photoelectrochemical performance of hematite (α-Fe2O3) electrodes by cadmium incorporation. Appl. Catal. B Environ. 2011, 110, 207–215. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Son, S.I.; Van, T.K.; Kang, Y.S. Preparation of α-Fe2O3 films by electrodeposition and photodeposition of Co–Pi on them to enhance their photoelectrochemical properties. RSC Adv. 2015, 5, 36307–36314. [Google Scholar] [CrossRef]
- Rahman, G.; Joo, O.-S. Electrodeposited nanostructured α-Fe2O3 thin films for solar water splitting: Influence of Pt doping on photoelectrochemical performance. Mater. Chem. Phys. 2013, 140, 316–322. [Google Scholar] [CrossRef]
- Chai, H.; Wang, P.; Wang, T.; Gao, L.; Li, F.; Jin, J. Surface Reconstruction of Cobalt Species on Amorphous Cobalt Silicate-Coated Fluorine-Doped Hematite for Efficient Photoelectrochemical Water Oxidation. ACS Appl. Mater. Interfaces 2021, 13, 47572–47580. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, Z. Simultaneous non-metal doping and cocatalyst decoration for efficient photoelectrochemical water splitting on hematite photoanodes. Electrochim. Acta 2018, 282, 48–55. [Google Scholar] [CrossRef]
- Kumar, V.; Ahlawat, D.S.; AarifUl Islam, S.; Singh, A. Ce doping induced modifications in structural, electrical and magnetic behaviour of hematite nanoparticles. Mater. Sci. Eng. B 2021, 272, 115327. [Google Scholar] [CrossRef]
- Petran, A.; Radu, T.; Borodi, G.; Nan, A.; Suciu, M.; Turcu, R. Effects of rare earth doping on multi-core iron oxide nanoparticles properties. Appl. Surf. Sci. 2018, 428, 492–499. [Google Scholar] [CrossRef]
- Wang, D.; Jin, L.; Li, Y.; Yao, D.; Wang, J.; Hu, H. Upgrading of vacuum residue with chemical looping partial oxidation over Ce doped Fe2O3. Energy 2018, 162, 542–553. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.; Si, G.; Li, Y.; Zhang, S.; Deng, X.; Xu, X. Oxygen vacancy defects engineering on Ce-doped α-Fe2O3 gas sensor for reducing gases. Sens. Actuators B Chem. 2020, 302, 127165. [Google Scholar] [CrossRef]
- Hou, Y.; Zuo, F.; Dagg, A.; Feng, P. Visible light-driven alpha-Fe2O3 nanorod/graphene/BiV1-xMoxO4 core/shell heterojunction array for efficient photoelectrochemical water splitting. Nano Lett. 2012, 12, 6464–6473. [Google Scholar] [CrossRef]
- Hsu, Y.K.; Chen, Y.C.; Lin, Y.G. Novel ZnO/Fe2O3 Core-Shell Nanowires for Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2015, 7, 14157–14162. [Google Scholar] [CrossRef]
- Fathabadi, M.; Qorbani, M.; Sabbah, A.; Quadir, S.; Huang, C.-Y.; Chen, K.-H.; Chen, L.-C.; Naseri, N. An ultrathin amorphous defective co-doped hematite passivation layer derived via an in situ electrochemical method for durable photoelectrochemical water oxidation. J. Mater. Chem. A 2022, 10, 16655–16665. [Google Scholar] [CrossRef]
- Qiu, P.; Li, F.; Zhang, H.; Wang, S.; Jiang, Z.; Chen, Y. Photoelectrochemical performance of α-Fe2O3@NiOOH fabricated with facile photo-assisted electrodeposition method. Electrochim. Acta 2020, 358, 136847. [Google Scholar] [CrossRef]
- Zhang, H.; Park, J.H.; Byun, W.J.; Song, M.H.; Lee, J.S. Activating the surface and bulk of hematite photoanodes to improve solar water splitting. Chem. Sci. 2019, 10, 10436–10444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.W.; Jiang, X.; Chen, Y.X.; Lin, S.W.; Lu, C.Z. N, P Self-Doped Porous Carbon Material Derived from Lotus Pollen for Highly Efficient Ethanol-Water Mixtures Photocatalytic Hydrogen Production. Nanomaterials 2022, 12, 1744. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Zhang, H.; Pan, Q.; Bian, J.; Chen, Z.; Cheng, C. 3D ordered urchin-like TiO2@Fe2O3 arrays photoanode for efficient photoelectrochemical water splitting. Appl. Surf. Sci. 2019, 470, 668–676. [Google Scholar] [CrossRef]
- Kim, S.; An, E.; Oh, I.; Hwang, J.B.; Seo, S.; Jung, Y.; Park, J.-C.; Choi, H.; Choi, C.H.; Lee, S. CeO2 nanoarray decorated Ce-doped ZnO nanowire photoanode for efficient hydrogen production with glycerol as a sacrificial agent. Catal. Sci. Technol. 2022, 12, 5517–5523. [Google Scholar] [CrossRef]
- Senthilkumar, P.; Jency, D.A.; Kavinkumar, T.; Dhayanithi, D.; Dhanuskodi, S.; Umadevi, M.; Manivannan, S.; Giridharan, N.V.; Thiagarajan, V.; Sriramkumar, M.; et al. Built-in Electric Field Assisted Photocatalytic Dye Degradation and Photoelectrochemical Water Splitting of Ferroelectric Ce Doped BaTiO3 Nanoassemblies. ACS Sustain. Chem. Eng. 2019, 7, 12032–12043. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, S.; Shi, R.; Wang, W.; Liang, K. Visible light active Ce/Ce2O/CeO2/TiO2 nanotube arrays for efficient hydrogen production by photoelectrochemical water splitting. Int. J. Hydrogen Energy 2016, 41, 5437–5444. [Google Scholar] [CrossRef]
- Radu, T.; Iacovita, C.; Benea, D.; Turcu, R. X-ray Photoelectron Spectroscopic Characterization of Iron Oxide Nanoparticles. Appl. Surf. Sci. 2017, 405, 337–343. [Google Scholar] [CrossRef]
- Tang, D.; Zhang, G. Efficient removal of fluoride by hierarchical Ce–Fe bimetal oxides adsorbent: Thermodynamics, kinetics and mechanism. Chem. Eng. J. 2016, 283, 721–729. [Google Scholar] [CrossRef]
- Mukhopadhyay, K.; Ghosh, U.C.; Sasikumar, P. Enhanced capacity of fluoride scavenging from contaminated water by nano-architectural reorientation of cerium-incorporated hydrous iron oxide with graphene oxide. Environ. Sci. Pollut. Res. Int. 2019, 26, 26112–26133. [Google Scholar] [CrossRef]
- Suganthi, N.; Pushpanathan, K. Effect of phase transition on room temperature ferromagnetism in cerium doped ZnS nanorods. Trans. Nonferrous Met. Soc. China 2019, 29, 811–820. [Google Scholar] [CrossRef]
- Randles, J.E.B. Kinetics of rapid electrode reactions. Discuss. Faraday Soc. 1947, 1, 11–19. [Google Scholar] [CrossRef]
- Qiu, P.; Yang, H.; Yang, L.; Wang, Q.; Ge, L. Solar water splitting with nanostructured hematite: The role of annealing-temperature. Electrochim. Acta 2018, 266, 431–440. [Google Scholar] [CrossRef]
- Gelderman, K.; Lee, L.; Donne, S.W. Flat-Band Potential of a Semiconductor: Using the Mott–Schottky Equation. J. Chem. Educ. 2007, 84, 685. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.-P.; Zhu, M.-L.; Shi, H.-Y.; Zhou, Q.-Q.; Chen, R.; Lin, S.-W.; Tong, M.-H.; Ji, M.-H.; Jiang, X.; Liao, C.-X.; et al. Cerium-Doped Iron Oxide Nanorod Arrays for Photoelectrochemical Water Splitting. Molecules 2022, 27, 9050. https://doi.org/10.3390/molecules27249050
Zhao H-P, Zhu M-L, Shi H-Y, Zhou Q-Q, Chen R, Lin S-W, Tong M-H, Ji M-H, Jiang X, Liao C-X, et al. Cerium-Doped Iron Oxide Nanorod Arrays for Photoelectrochemical Water Splitting. Molecules. 2022; 27(24):9050. https://doi.org/10.3390/molecules27249050
Chicago/Turabian StyleZhao, Hai-Peng, Mei-Ling Zhu, Hao-Yan Shi, Qian-Qian Zhou, Rui Chen, Shi-Wei Lin, Mei-Hong Tong, Ming-Hao Ji, Xia Jiang, Chen-Xing Liao, and et al. 2022. "Cerium-Doped Iron Oxide Nanorod Arrays for Photoelectrochemical Water Splitting" Molecules 27, no. 24: 9050. https://doi.org/10.3390/molecules27249050
APA StyleZhao, H. -P., Zhu, M. -L., Shi, H. -Y., Zhou, Q. -Q., Chen, R., Lin, S. -W., Tong, M. -H., Ji, M. -H., Jiang, X., Liao, C. -X., Chen, Y. -X., & Lu, C. -Z. (2022). Cerium-Doped Iron Oxide Nanorod Arrays for Photoelectrochemical Water Splitting. Molecules, 27(24), 9050. https://doi.org/10.3390/molecules27249050