Development of an Inhibition Enzyme-Linked Immunosorbent Assay (ELISA) Prototype for Detecting Cytotoxic Three-Finger Toxins (3FTxs) in African Spitting Cobra Venoms
Abstract
:1. Introduction
2. Results
2.1. Optimization of ELISA Parameters
2.2. Determination of ELISA Cut-Off Point
2.3. Evaluation of the Developed Prototype
2.3.1. Inhibition ELISA for Detecting Three-Finger Toxins in Crude N. ashei and Other Venoms
2.3.2. Inhibition ELISA for Detecting 3FTx Proteins in Spiked Samples
2.3.3. Inhibition ELISA for Detecting 3FTxs in Mice Challenged with N. ashei Venom
3. Discussion
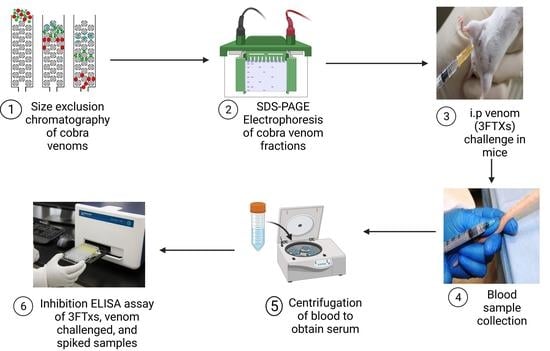
4. Materials and Methods
4.1. Snake Venoms
4.2. Animals
4.3. Ethical and Institutional Approvals
4.4. Quantification of Protein Content of Venoms
4.5. Optimization of ELISA Parameters
4.6. Determination of Specificity and Sensitivity of Inhibition ELISA
4.7. Inhibition ELISA for Detecting Three-Finger Toxins in Crude N. ashei Venom
Testing Inhibition ELISA for Detecting 3FTxs in Other Naja sp. and Non-Naja sp. Venoms
4.8. Detection of Three-Finger Toxins in Spiked Samples
4.9. Detection of Three-Finger Toxins in N. ashei Venom-Challenged Mice
4.10. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gutiérrez, J.M.; Calvete, J.; Habib, A.G.; Harrison, R.A.; Williams, D.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Snakebite Envenoming. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/snakebite-envenoming (accessed on 15 January 2022).
- Ministry of Health. Guidelines for Prevention, Diagnosis and Management of Snakebite Envenoming in Kenya; Ministry of Health: Nairobi, Kenya, 2019.
- Wüster, W.; Broadley, D.G. Get an eyeful of this: A new species of giant spitting cobra from eastern and north-eastern Africa (Squamata: Serpentes: Elapidae: Naja). Zootaxa 2007, 1532, 51–68. [Google Scholar] [CrossRef]
- Hus, K.K.; Buczkowicz, J.; Petrilla, V.; Petrillová, M.; Łyskowski, A.; Legáth, J.; Bocian, A. First Look at the Venom of Naja ashei. Molecules 2018, 23, 609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hus, K.K.; Marczak, Ł.; Petrilla, V.; Petrillová, M.; Legáth, J.; Bocian, A. Different Research Approaches in Unraveling the Venom Proteome of Naja ashei. Biomolecules 2020, 10, 1282. [Google Scholar] [CrossRef] [PubMed]
- Okumu, M.O.; Mbaria, J.M.; Gikunju, J.K.; Mbuthia, P.G.; Madadi, V.O.; Ochola, F.O. Enzymatic activity and brine shrimp lethality of venom from the large brown spitting cobra (Naja ashei) and its neutralization by antivenom. BMC Res. Notes 2020, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Petras, D.; Sanz, L.; Segura, A.; Herrera, M.; Villalta, M.; Solano, D.; Vargas, M.; León, G.; Warrell, D.A.; Theakston, R.D.G.; et al. Snake Venomics of African Spitting Cobras: Toxin Composition and Assessment of Congeneric Cross-Reactivity of the Pan-African EchiTAb-Plus-ICP Antivenom by Antivenomics and Neutralization Approaches. J. Proteome Res. 2011, 10, 1266–1280. [Google Scholar] [CrossRef]
- Bermúdez-Méndez, E.; Fuglsang-Madsen, A.; Føns, S.; Lomonte, B.; Gutiérrez, J.M.; Laustsen, A.H. Innovative Immunization Strategies for Antivenom Development. Toxins 2018, 10, 452. [Google Scholar] [CrossRef] [Green Version]
- Kini, R.M.; Doley, R. Structure, function and evolution of three-finger toxins: Mini proteins with multiple targets. Toxicon 2010, 56, 855–867. [Google Scholar] [CrossRef]
- Bernardoni, J.L.; de Sousa, L.; Wermelinger, L.S.; Lopes, A.S.; Prezoto, B.C.; Serrano, S.M.T.; Zingali, R.B.; Moura-Da-Silva, A.M. Functional Variability of Snake Venom Metalloproteinases: Adaptive Advantages in Targeting Different Prey and Implications for Human Envenomation. PLoS ONE 2014, 9, e109651. [Google Scholar] [CrossRef] [Green Version]
- Williams, H.F.; Layfield, H.J.; Vallance, T.; Patel, K.; Bicknell, A.B.; Trim, S.A.; Vaiyapuri, S. The Urgent Need to Develop Novel Strategies for the Diagnosis and Treatment of Snakebites. Toxins 2019, 11, 363. [Google Scholar] [CrossRef] [Green Version]
- Williams, H.F.; Vaiyapuri, R.; Gajjeraman, P.; Hutchinson, G.; Gibbins, J.M.; Bicknell, A.B.; Vaiyapuri, S. Challenges in diagnosing and treating snakebites in a rural population of Tamil Nadu, India: The views of clinicians. Toxicon 2017, 130, 44–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulfone, T.C.; Samuel, S.P.; Bickler, P.E.; Lewin, M.R. Developing Small Molecule Therapeutics for the Initial and Adjunctive Treatment of Snakebite. J. Trop. Med. 2018, 2018, 4320175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvanayagam, Z.; Gopalakrishnakone, P. Tests for detection of snake venoms, toxins and venom antibodies: Review on recent trends (1987–1997). Toxicon 1999, 37, 565–586. [Google Scholar] [CrossRef]
- Kulawickrama, S.; O’Leary, M.; Hodgson, W.; Brown, S.; Jacoby, T.; Davern, K.; Isbister, G. Development of a sensitive enzyme immunoassay for measuring taipan venom in serum. Toxicon 2010, 55, 1510–1518. [Google Scholar] [CrossRef]
- Van Dong, L. Immunogenicity of venoms from four common snakes in the South of Vietnam and development of ELISA kit for venom detection. J. Immunol. Methods 2003, 282, 13–31. [Google Scholar] [CrossRef]
- Morais, V.; Berasain, P.; Ifrán, S.; Carreira, S.; Tortorella, M.N.; Negrín, A.; Massaldi, H. Humoral immune responses to venom and antivenom of patients bitten by Bothrops snakes. Toxicon 2012, 59, 315–319. [Google Scholar] [CrossRef]
- Norris, R.L.; Pfalzgraf, R.R.; Laing, G. Death following coral snake bite in the United States—First documented case (with ELISA confirmation of envenomation) in over 40 years. Toxicon 2009, 53, 693–697. [Google Scholar] [CrossRef]
- Hung, D.-Z.; Liau, M.-Y.; Lin-Shiau, S.-Y. The clinical significance of venom detection in patients of cobra snakebite. Toxicon 2003, 41, 409–415. [Google Scholar] [CrossRef]
- Warrell, D.A. Snake bite. Lancet 2010, 375, 77–88. [Google Scholar] [CrossRef]
- Hung, D.Z.; Lin, J.H.; Mo, J.F.; Huang, C.F.; Liau, M.Y. Rapid diagnosis of Naja atrasnakebites. Clin. Toxicol. 2014, 52, 187–191. [Google Scholar] [CrossRef]
- CSL. Snake venom detection Kit (SVDK)-detection and identification of snake. Clin. Toxicol. 2017, 55, 1–19. [Google Scholar]
- Sharma, N.; Hanif, S.; Upadhyay, D.; Chhikara, M.K. Inhibition ELISA as a putative tool for the identification and quantification of meningococcal A and X polysaccharides at various stages of vaccine development. J. Immunol. Methods 2019, 473, 112634. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, A.H.; Engmark, M.; Clouser, C.; Timberlake, S.; Vigneault, F.; Gutiérrez, J.M.; Lomonte, B. Exploration of immunoglobulin transcriptomes from mice immunized with three-finger toxins and phospholipases A2 from the Central American coral snake, Micrurus nigrocinctus. PeerJ 2017, 5, e2924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosoda, H.; Takasaki, W.; Oe, T.; Tsukamoto, R.; Nambara, T. A comparison of chromogenic substrates for horseradish peroxidase as a label in steroid enzyme immunoassay. Chem. Pharm. Bull. 1986, 34, 4177–4182. [Google Scholar] [CrossRef] [Green Version]
- Goka, A.K.J.; Farthing, M.J. The Use of 3, 3′, 5, 5′-Tetramethylbenzidine as a Peroxidase Substrate in Microplate Enzyme-Linked Immunosorbent Assay. J. Immunoass. 1987, 8, 29–41. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Lomonte, B.; Lohse, B.; Fernández, J.; Gutiérrez, J.M. Unveiling the nature of black mamba (Dendroaspis polylepis) venom through venomics and antivenom immunoprofiling: Identification of key toxin targets for antivenom development. J. Proteom. 2015, 119, 126–142. [Google Scholar] [CrossRef]
- Blanchet, G.; Collet, G.; Mourier, G.; Gilles, N.; Fruchart-Gaillard, C.; Marcon, E.; Servent, D. Polypharmacology profiles and phylogenetic analysis of three-finger toxins from mamba venom: Case of aminergic toxins. Biochimie 2014, 103, 109–117. [Google Scholar] [CrossRef]
- Casasola, A.; Ramos-Cerrillo, B.; de Roodt, A.R.; Saucedo, A.C.; Chippaux, J.-P.; Alagón, A.; Stock, R.P. Paraspecific neutralization of the venom of African species of cobra by an equine antiserum against Naja melanoleuca: A comparative study. Toxicon 2009, 53, 602–608. [Google Scholar] [CrossRef]
- Sánchez, A.; Herrera, M.; Villalta, M.; Solano, D.; Segura, Á.; Lomonte, B.; Gutiérrez, J.M.; León, G.; Vargas, M. Proteomic and toxinological characterization of the venom of the South African Ringhals cobra Hemachatus haemachatus. J. Proteom. 2018, 181, 104–117. [Google Scholar] [CrossRef]
- Armbruster, D.A.; Pry, T. Limit of Blank, Limit of Detection and Limit of Quantitation. Clin. Biochem. Rev. 2008, 29 (Suppl. S1), S49–S52. [Google Scholar]
- Classen, D.C.; Morningstar, J.M.; Shanley, J.D. Detection of antibody to murine cytomegalovirus by enzyme-linked immunosorbent and indirect immunofluorescence assays. J. Clin. Microbiol. 1987, 25, 600–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lardeux, F.; Torrico, G.; Aliaga, C. Calculation of the ELISA’s cut-off based on the change-point analysis method for detection of Trypanosoma cruzi infection in Bolivian dogs in the absence of controls. Memórias Inst. Oswaldo Cruz 2016, 111, 501–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.C.; Yu, J.S.; Wang, P.J.; Hsiao, Y.C.; Liu, C.H.; Chen, Y.C.; Lai, P.F.; Hsu, C.P.; Fann, W.C.; Lin, C.C. Development of sandwich ELISA and lateral flow strip assays for diagnosing clinically significant snakebite in Taiwan. PLOS Negl. Trop. Dis. 2018, 12, e0007014. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, I.K.; Dixit, P.P.; Pawade, B.S.; Waykar, I.G. Development of dot-ELISA for the detection of venoms of major Indian venomous snakes. Toxicon 2017, 139, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Sakai, A.; Kouda, T.; Sawai, Y. Detection of Agkistrodon b. blomho i venom in serum of mice by enzyme-linked immunosorbent assay (ELISA). Snake 1988, 20, 25–29. [Google Scholar]
- Selvanayagam, Z. Studies on Ehretia Buxifolia, a Herbal Antidote for Echis Carinatus Envenomation and Development of ELISA for Detection of Snake Venoms. Ph.D. Thesis, University of Madras, Madras, India, 1995. [Google Scholar]
- Dyba, B.; Barbasz, A.; Czy, A.; Hus, K.K. Effects of 3FTx Protein Fraction from Naja ashei Venom on the Model and Native Membranes: Recognition and Implications for the Mechanisms of Toxicity. Molecules 2021, 26, 2164. [Google Scholar] [CrossRef]
- ThermoFisher Scientific. Pierce BCA Protein Assay Kit User Guide. Illinois, USA. 2020. Available online: https://www.thermofisher.com/bca (accessed on 20 March 2021).
- Islam, M.; Jones, R. An enzyme-linked immunosorbent assay for measuring antibody titre against avian reovirus using a single dilution of serum. Avian Pathol. 1988, 17, 411–425. [Google Scholar] [CrossRef]
- WHO. WHO Expert Committee on Biological Standardization; World Health Organization: Geneva, Switzerland, 2017.




| Inhibitor Concentration (µg/mL) | Naja ashei | Naja nigricollis | Naja haje | Bitis arietans | Dendroapsis polylepsis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OD | % Inhibition | OD | % Inhibition | OD | % Inhibition | OD | % Inhibition | OD | % Inhibition | |
| 6.000 | 0.094 | 76.90 | 0.085 | 77.68 | 0.088 | 79.75 | 0.474 | 7.06 | 0.458 | 8.77 |
| 2.000 | 0.118 | 71.13 | 0.087 | 77.01 | 0.091 | 79.17 | 0.464 | 9.02 | 0.439 | 12.46 |
| 0.667 | 0.174 | 57.25 | 0.098 | 74.24 | 0.098 | 77.45 | 0.454 | 11.08 | 0.458 | 8.67 |
| 0.222 | 0.259 | 36.49 | 0.125 | 66.97 | 0.113 | 73.99 | 0.467 | 8.53 | 0.465 | 7.28 |
| 0.074 | 0.295 | 27.64 | 0.177 | 53.37 | 0.131 | 69.85 | 0.471 | 7.65 | 0.445 | 11.27 |
| 0.025 | 0.319 | 21.62 | 0.252 | 33.42 | 0.182 | 58.11 | 0.447 | 12.45 | 0.434 | 13.46 |
| 0.008 | 0.331 | 18.80 | 0.299 | 21.00 | 0.260 | 40.28 | 0.447 | 12.35 | 0.424 | 15.55 |
| NAC | 0.407 | 0.379 | 0.435 | 0.510 | 0.502 | |||||
| % Inhibition for Determining Sensitivity of Inhibition ELISA | ||||
|---|---|---|---|---|
| N. ashei Venom | Negative Controls (n = 12) | |||
| Conc (µg/mL) | Replicate 1 | Replicate 2 | ||
| 27.00 | 76.69 | 78.68 | 49.60 | 49.70 |
| 9.00 | 75.28 | 77.22 | 47.71 | 48.91 |
| 3.00 | 69.62 | 76.04 | 46.52 | 45.97 |
| 1.00 | 64.39 | 70.54 | 26.80 | 20.83 |
| 0.33 | 54.18 | 56.96 | 25.09 | 25.82 |
| 0.11 | 45.02 | 44.95 | 20.46 | 21.92 |
| 0.04 | 34.44 | 38.55 | - | - |
| Inhibitor Concentration (µg/mL) | OD (492 nm) | % Inhibition |
|---|---|---|
| 27.00 | 0.380 | 77.70 |
| 9.00 | 0.404 | 76.29 |
| 3.00 | 0.461 | 72.95 |
| 1.00 | 0.552 | 67.61 |
| 0.33 | 0.756 | 55.63 |
| 0.11 | 0.938 | 44.95 |
| 0.04 | 1.081 | 36.56 |
| No antigen control (NAC) | 1.704 | - |
| F | 19.14 |
| p value | <0.0001 |
| p value summary | **** |
| Significant diff. among means (p < 0.05)? | Yes |
| R squared | 0.7185 |
| Tukey’s Multiple Comparisons Test | Adjusted p Value | Significant? |
|---|---|---|
| N. ashei vs. N. nigricollis | 0.5409 | No |
| N. ashei vs. N. haje | 0.0649 | No |
| N. ashei vs. B. arietans | 0.0034 | Yes |
| N. ashei vs. D. polylepsis | 0.0051 | Yes |
| N. nigricollis vs. N. haje | 0.7312 | No |
| N. nigricollis vs. B. arietans | <0.0001 | Yes |
| N. nigricollis vs. D. polylepsis | <0.0001 | Yes |
| N. haje vs. B. arietans | <0.0001 | Yes |
| N. haje vs. D. polylepsis | <0.0001 | Yes |
| B. arietans vs. D. polylepsis | 0.9999 | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manson, E.Z.; Mutinda, K.C.; Gikunju, J.K.; Bocian, A.; Hus, K.K.; Petrílla, V.; Legáth, J.; Kimotho, J.H. Development of an Inhibition Enzyme-Linked Immunosorbent Assay (ELISA) Prototype for Detecting Cytotoxic Three-Finger Toxins (3FTxs) in African Spitting Cobra Venoms. Molecules 2022, 27, 888. https://doi.org/10.3390/molecules27030888
Manson EZ, Mutinda KC, Gikunju JK, Bocian A, Hus KK, Petrílla V, Legáth J, Kimotho JH. Development of an Inhibition Enzyme-Linked Immunosorbent Assay (ELISA) Prototype for Detecting Cytotoxic Three-Finger Toxins (3FTxs) in African Spitting Cobra Venoms. Molecules. 2022; 27(3):888. https://doi.org/10.3390/molecules27030888
Chicago/Turabian StyleManson, Ernest Z., Kyama C. Mutinda, Joseph K. Gikunju, Aleksandra Bocian, Konrad K. Hus, Vladimír Petrílla, Jaroslav Legáth, and James H. Kimotho. 2022. "Development of an Inhibition Enzyme-Linked Immunosorbent Assay (ELISA) Prototype for Detecting Cytotoxic Three-Finger Toxins (3FTxs) in African Spitting Cobra Venoms" Molecules 27, no. 3: 888. https://doi.org/10.3390/molecules27030888
APA StyleManson, E. Z., Mutinda, K. C., Gikunju, J. K., Bocian, A., Hus, K. K., Petrílla, V., Legáth, J., & Kimotho, J. H. (2022). Development of an Inhibition Enzyme-Linked Immunosorbent Assay (ELISA) Prototype for Detecting Cytotoxic Three-Finger Toxins (3FTxs) in African Spitting Cobra Venoms. Molecules, 27(3), 888. https://doi.org/10.3390/molecules27030888