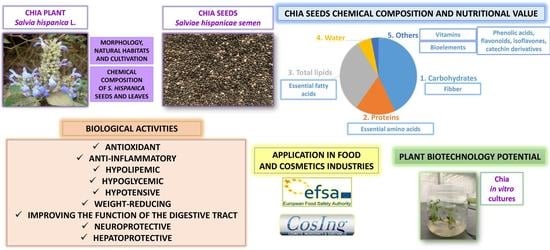
The Current State of Knowledge on Salvia hispanica and Salviae hispanicae semen (Chia Seeds)
Abstract
:1. Introduction
2. Morphology, Natural Habitats, and Cultivation
3. Bioactive Components of S. hispanica Seeds
4. Chemical Composition of S. hispanica Leaves
5. Biological Activities of S. hispanica Seeds Confirmed by Scientific Research
5.1. Hypoglycemic, Hypotensive, Hypolipemic, Hepatoprotective, and Fat-Reducing Effects
5.1.1. Animal Model Studies
5.1.2. Clinical Studies
5.2. Antioxidant and Neuroprotective Effect
5.3. Action against Kidney Stones
5.4. Improving the Function of the Digestive Tract
6. Application of S. hispanica in Cosmetology
7. Applications of Chia Seeds in the Food Industry
8. Plant Biotechnology Studies of S. hispanica
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lu, Y.; Foo, L.Y. Polyphenolics in Salvia. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef]
- Segura-Campos, M.R.; Ciau-Solís, N.; Rosado-Rubio, G.; Chel-Guerrero, L.; Betancur-Ancona, D. Chemical and Functional properties of chia seed (Salvia hispanica L.) gum. Int. J. Food Sci. 2014, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cahill, J.P. Ethnobotany of chia, Salvia hispanica L. (Lamiaceae). Econ. Bot. 2003, 57, 604–618. [Google Scholar] [CrossRef]
- Ayerza, R.; Coates, W. Chia: Rediscovering a Forgotten Crop of the Aztecs; University of Arizona Press: Tucson, AZ, USA, 2005. [Google Scholar]
- Uribe, J.A.R.; Perez, J.I.N.; Kauil, H.C.; Rubio, G.R.; Alcocer, C.G. Extraction of oil from chia seeds with supercritical CO2. J. Supercrit. Fluids. 2011, 56, 174–178. [Google Scholar] [CrossRef]
- Enes, B.N.; Moreira, L.P.D.; Silva, B.P.; Grancieri, M.; Lúcio, H.G.; Venâncio, V.P.; Mertens-Talcott, S.U.; Rosa, C.O.B.; Martino, H.S.D. Chia seed (Salvia hispanica L.) effects and their molecular mechanisms on unbalanced diet experimental studies: A systematic review. J. Food Sci. 2020, 85, 226–239. [Google Scholar] [CrossRef] [Green Version]
- Das, A. Advances in chia seed research. Adv. Biotechnol. Microbiol. 2017. [Google Scholar] [CrossRef]
- Norlaily, M.; Yeap, A.K.S.; Wan, Y.H.; Boon, K.B.; Sheau, W.T.; Soon, G.T. The promising future of chia, Salvia hispanica L. J. Biomed. Biotechnol. 2012. [Google Scholar] [CrossRef] [Green Version]
- Hrnčič, K.; IvanovskI, C. Chia seeds (Salvia hispanica L.): An overview—Phytochemical profile, isolation methods, and application. Molecules 2019, 25, 11. [Google Scholar] [CrossRef] [Green Version]
- European Directorate for the Quality of Medicines. European Pharmacopoeia 9.0; European Directorate for the Quality of Medicines: Strasbourg, France, 2017. [Google Scholar]
- Perry, E.K.; Pickering, A.T.; Wang, W.W.; Houghton, P.J.; Perry, N.S.L. Medicinal plants and Alzheimer’s disease: From ethnobotany to phytotherapy. J. Pharm. Pharmacol. 2010, 51, 527–534. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Lopresti, A.L. Salvia (sage): A review of its potential cognitive-enhancing and protective effects. Drugs R D 2017, 17, 53–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porres-Martinez, M.; Accame, M.; Gómez-Serranillos, M. Pharmacological activity of Salvia lavandulifolia and chemical components of its essential oil. A Review. Lazaroa 2013, 34, 237–254. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.J.; Kim, K.Y.; Kang, P.; Lee, H.S.; Seo, G.H. Effects of Salvia sclarea on chronic immobilization stress induced endothelial dysfunction in rats. BMC Complement. Altern. Med. 2014, 14, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, C.Y.; Ming, Q.L.; Rahman, K.; Han, T.; Qin, L.P. Salvia miltiorrhiza: Traditional medicinal uses, chemistry, and pharmacology. Chin. J. Nat. Med. 2015, 13, 163–182. [Google Scholar] [CrossRef]
- Baldivia, A.S. A historical review of the scientific and common nomenclature associated with chia: From Salvia hispanica to Salvia mexicana and chian to salba. Agric. Res. Technol. Open Access J. 2018, 18, 1–8. [Google Scholar] [CrossRef]
- Cafligueral, S.; Iglesias, J.; Hamburger, M.; Hostettmann, K. Phenolic constituents of Salvia lavanduljfolia ssp. lavandulifolia. Phytochemistry 1989. [Google Scholar]
- Craig, R.; Sons, M. Application for Approval of Whole Chia (Salvia hispanica L.) Seed and Ground Whole Seed as Novel Food Ingredient. 2004. Available online: https://acnfp.food.gov.uk/sites/default/files/mnt/drupal_data/sources/files/multimedia/pdfs/chiaapplication.pdf (accessed on 3 December 2021).
- Ayerza, R.; Coates, W. Protein content, oil content and fatty acid profiles as potential criteria to determine the origin of commercially grown chia (Salvia hispanica L.). Ind. Crops Prod. 2011, 34, 1366–1371. [Google Scholar] [CrossRef]
- Coates, W. Whole and ground chia (Salvia hispanica L.) seeds, chia oil—Effects on plasma lipids and fatty acids. In Nuts and Seeds in Health and Disease Prevention; Academic Press: London, UK, 2011; pp. 309–315. [Google Scholar]
- Suri, S.; Passi, S.J.; Goyat, J. Chia seed (Salvia hispanica L.)—A new age functional food. Int. J. Adv. Technol. Eng. Sci. 2016, 4, 286–299. [Google Scholar]
- Muñoz, L.; Cobos, A.; Diaz, O.; Aguilera, J. Chia seeds: Microstructure, mucilage extraction and hydration. J. Food Eng. 2012, 108, 216–224. [Google Scholar] [CrossRef]
- The Plant List—A Working List of All Plant Species. Available online: http://www.theplantlist.org/ (accessed on 12 September 2020).
- World Flora Online. Available online: http://www.worldfloraonline.org/taxon/wfo-0000301201#localNames (accessed on 12 September 2020).
- European Directorate for the Quality of Medicines. European Pharmacopoeia 10.0; European Directorate for the Quality of Medicines: Strasbourg, France, 2020. [Google Scholar]
- Nieman, C.; Cayea, E.J.; Austin, M.D.; Henson, D.A.; McAnulty, F.J. Chia seed does not promote weight loss or alter disease risk factors in overweight adults. Nutr. Res. 2009, 29, 414–418. [Google Scholar] [CrossRef]
- Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of chia seeds (Salvia hispanica L.) as a novel food for extended uses pursuant to Regulation (EU) 2015/2283. EFSA J. 2019, 17, e05657. [Google Scholar] [CrossRef] [Green Version]
- Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of chia seeds (Salvia hispanica L.) powders, as novel foods, pursuant to Regulation (EU) 2015/2283. EFSA J. 2019, 17, e05716. [Google Scholar] [CrossRef] [PubMed]
- European Commission CosIng-Cosmetic Database. Available online: https://ec.europa.eu/growth/sectors/cosmetics/cosmetic-ingredient-database_en (accessed on 10 October 2021).
- Kang, Y.; Ha, W.; Liu, Y.-Q.; Ma, Y.; Fan, M.-M.; Ding, L.-S.; Zhang, S.; Li, B.-J. PH-responsive polymer–drug conjugates as multifunctional micelles for cancer-drug delivery. Nanotechnology 2014, 25, 335101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulczyński, B.; Kobus-Cisowska, J.; Taczanowski, M.; Kmiecik, D.; Gramza-Michałowska, A. The chemical composition and nutritional value of chia seeds—current state of knowledge. Nutrients 2019, 11, 1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabail, R.; Khan, M.R.; Mehwish, H.F.; Rajoka, M.S.R.; Lorenzo, J.M.; Kieliszek, M.; Khalid, A.R.; Shabbir, M.A. An overview of chia seed (Salvia hispanica L.) bioactive peptides’ derivation and utilization as an emerging nutraceutical food. Front. Biosci. 2021, 26, 643. [Google Scholar] [CrossRef]
- Grancieri, M.; Martino, H.S.D.; Gonzalez de Mejia, E. Protein digests and pure peptides from chia seed prevented adipogenesis and inflammation by inhibiting PPARγ and NF-κB pathways in 3T3L-1 adipocytes. Nutrients 2021, 13, 176. [Google Scholar] [CrossRef]
- Miranda-Ramos, K.C.; Haros, C.M. Combined effect of chia, quinoa and amaranth incorporation on the physico-chemical, nutritional and functional quality of fresh bread. Foods 2020, 9, 1859. [Google Scholar] [CrossRef]
- Saleemnazir, U.; Ahmad, M.; Ajmal Shah, M.; Fareeha, A.; Ahmad, B. Anti-urolithiatic activity of Salvia hispanica L. seeds in ethylene glycol induced urolithiasis rat’s model. Health Sci. 2020, 92, e20200067. [Google Scholar] [CrossRef]
- Miranda-Ramos, K.; Millán-Linares, M.C.; Haros, C.M. Effect of chia as breadmaking ingredient on nutritional quality, mineral availability, and glycemic index of bread. Foods 2020, 9, 663. [Google Scholar] [CrossRef] [PubMed]
- Motyka, S.; Ekiert, H.; Szopa, A. Chemical composition, biological activity and utilization of chia seeds (Salviae hispanicae semen). Farm. Pol. 2021, 77, 651–661. [Google Scholar] [CrossRef]
- Kuznetcova, D.V.; Linder, M.; Jeandel, C.; Paris, C.; Desor, F.; Baranenko, D.A.; Nadtochii, L.A.; Arab-Tehrany, E.; Yen, F.T. Nanoliposomes and nanoemulsions based on chia seed lipids: Preparation and characterization. Int. J. Mol. Sci. 2020, 21, 9079. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsion-based oral delivery systems for lipophilic bioactive components: Nutraceuticals and pharmaceuticals. Ther. Deliv. 2013, 4, 841–857. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Teng, J.; Hu, X.; Wang, M.; Tao, N. Fabrication of chia (Salvia hispanica L.) seed oil nanoemulsions using different emulsifiers. J. Food Process. Preserv. 2018, 42, e13416. [Google Scholar] [CrossRef]
- Ferreira, C.D.; Nunes, I.L. Oil nanoencapsulation: Development, application, and incorporation into the food market. Nanoscale Res. Lett. 2019, 14, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chau, C.-F.; Wu, S.-H.; Yen, G.-C. The development of regulations for food nanotechnology. Trends Food Sci. Technol. 2007, 18, 269–280. [Google Scholar] [CrossRef]
- De Vos, P.; Faas, M.M.; Spasojevic, M.; Sikkema, J. Encapsulation for preservation of functionality and targeted delivery of bioactive food components. Int. Dairy J. 2010, 20, 292–302. [Google Scholar] [CrossRef]
- Rodríguez, J.; Martín, M.J.; Ruiz, M.A.; Clares, B. Current encapsulation strategies for bioactive oils: From alimentary to pharmaceutical perspectives. Food Res. Int. 2016, 83, 41–59. [Google Scholar] [CrossRef]
- Ullah, R.; Nadeem, M.; Khalique, A.; Imran, M.; Mehmood, S.; Javid, A.; Hussain, J. Nutritional and therapeutic perspectives of Chia (Salvia hispanica L.): A review. J. Food Sci. Technol. 2016, 53, 1750–1758. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, L.A.; Cobos, A.; Diaz, O.; Aguilera, J.M. Chia seed (Salvia hispanica): An ancient grain and a new functional food. Food Rev. Int. 2013, 29, 394–408. [Google Scholar] [CrossRef]
- Ulbricht, C.; Chao, W.; Nummy, K.; Rusie, E.; Tanguay-Colucci, S.; Iannuzzi, C.; Plammoottil, J.; Varghese, M.; Weissner, W. Chia (Salvia hispanica): A systematic review by the natural standard research collaboration. Rev. Recent Clin. Trials. 2009, 4, 168–174. [Google Scholar] [CrossRef]
- Bordin-Rodrigues, C.J. Management, bean and chia development in accordance with fertilization. Heliyon 2021, 7, e07316. [Google Scholar] [CrossRef]
- Benetoli da Silva, T.R.; de Melo, S.C.; Nascimento, A.B.; Ambrosano, L.; Bordin, J.C.; Alves, C.Z.; Secco, D.; Santos, R.F.; Gonçalves, A.C., Jr.; da Silva, G.D. Response of chia (Salvia hispanica) to sowing times and phosphorus rates over two crop cycles. Heliyon 2020, 6, e05051. [Google Scholar] [CrossRef] [PubMed]
- Huxley, A.J. The New RHS Dictionary of Gardening; Mac Millan Press: London, UK, 1992. [Google Scholar]
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113, 71–88. [Google Scholar] [CrossRef]
- Da Luz, J.M.R.; Nunes, M.D.; Paes, S.A.; Torres, D.P.; Silva, M.D. Lignocellulolytic enzyme production of Pleurotus ostreatus growth in agroindustrial wastes. Braz. J. Microbiol. 2012, 43, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, H.; Panchal, S.K.; Waanders, J.; Ward, L.; Brown, L. Lipid redistribution by α-linolenic acid-rich chia seed inhibits stearoyl-CoA desaturase-1 and induces cardiac and hepatic protection in diet-induced obese rats. J. Nutr. Biochem. 2012, 23, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, B.P.; Kolba, N.; Duarte Martino, H.S.; Hart, J.; Tako, E. Soluble extracts from chia seed (Salvia hispanica L.) affect brush border membrane functionality, morphology and intestinal bacterial populations in vivo (Gallus gallus). Nutrients 2019, 11, 2457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodoira, R.M.; Penci, M.C.; Ribotta, P.D.; Martínez, M.L. Chia (Salvia hispanica L.) oil stability: Study of the effect of natural antioxidants. Food Sci. Technol. 2017, 75, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Melo, D.; MacHado, T.B.; Oliveira, M.B.P.P. Chia seeds: An ancient grain trending in modern human diets. Food Funct. 2019, 10, 3068–3089. [Google Scholar] [CrossRef]
- Ciftci, O.N.; Przybylski, R.; Rudzińska, M. Lipid components of flax, perilla, and chia seeds. Eur. J. Lipid Sci. Technol. 2012, 114, 794–800. [Google Scholar] [CrossRef]
- Nitrayová, S.; Brestenský, M.; Heger, J.; Patráš, P.; Rafay, J.; Sirotkin, A. Amino acids and fatty acids profile of chia (Salvia hispanica L.) and flax (Linum usitatissimum L.) seed. Potravinarstvo 2014, 8, 267–275. [Google Scholar] [CrossRef]
- Peiretti, P.G.; Gai, F. Fatty acid and nutritive quality of chia (Salvia hispanica L.) seeds and plant during growth. Anim. Feed Sci. Technol. 2009, 148, 267–275. [Google Scholar] [CrossRef]
- Silveira Coelho, M.; de las Mercedes Salas-Mellado, M. Chemical characterization of chia (Salvia hispanica L.) for use in food products. J. Food Nutr. Res. 2014, 2, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Chávez, L.M.; Valdivia-López, M.d.l.A.; Aburto-Juárez, M.d.L.; Tecante, A. Chemical characterization of the lipid fraction of mexican chia seed (Salvia hispanica L.). Int. J. Food Prop. 2008, 11, 687–697. [Google Scholar] [CrossRef]
- Rossi, S.A.; Oliva, M.E.; Ferreira, M.R.; Chicco, A. Dietary chia seed induced changes in hepatic transcription factors and their target lipogenic and oxidative enzyme activities in dyslipidaemic insulin-resistant rats. Br. J. Nutr. 2013, 109, 1617–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdivia-López, M.Á.; Tecante, A. Chia (Salvia hispanica): A Review of Native Mexican Seed and its Nutritional and Functional Properties. Adv. Food Nutr. Res. 2015, 75, 53–75. [Google Scholar] [CrossRef]
- Reyes-Caudillo, E.; Tecante, A.; Valdivia, M. Dietary fibre content and antioxidant activity of phenolic compounds present in Mexican chia (Salvia hispanica L.) seeds. Food Chem. 2008, 107, 656–663. [Google Scholar] [CrossRef]
- Marineli, R.; Lenquiste, S.; Moraes, E.; Maróstica, M., Jr. Antioxidant potential of dietary chia seed and oil (Salvia hispanica L.) in diet-induced obese rats. Food Res. Int. 2015, 76, 666–674. [Google Scholar] [CrossRef]
- Peláez, P.; Orona-Tamayo, D.; Montes-Hernández, S.; Valverde, M.E.; Paredes-López, O.; Cibrián-Jaramillo, A. Comparative transcriptome analysis of cultivated and wild seeds of Salvia hispanica (chia). Sci. Rep. 2019, 9, 9761. [Google Scholar] [CrossRef]
- Bushway, A.A.; Belyea, P.R.; Bushway, R.J. Chia seed as a source of oil, polysaccharide, and protein. J. Food Sci. 1981, 46, 1349–1350. [Google Scholar] [CrossRef]
- Sandoval-Oliveros, M.R.; Paredes-López, O. Isolation and characterization of proteins from chia seeds (Salvia hispanica L.). J. Agric. Food Chem. 2013, 61, 193–201. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/1100610/nutrients (accessed on 12 November 2021).
- Martínez-Cruz, O.; Paredes-López, O. Phytochemical profile and nutraceutical potential of chia seeds (Salvia hispanica L.) by ultra high performance liquid chromatography. J. Chromatogr. A 2014, 1346, 43–48. [Google Scholar] [CrossRef]
- Pellegrini, M.; Lucas-Gonzalez, R.; Sayas-Barberá, E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Bioaccessibility of phenolic compounds and antioxidant capacity of chia (Salvia hispanica L.) seeds. Plant Foods Hum. Nutr. 2018, 73, 47–53. [Google Scholar] [CrossRef]
- Jin, F.; Nieman, D.C.; Sha, W.; Xie, G.; Qiu, Y.; Jia, W. Supplementation of milled chia seeds increases plasma ALA and EPA in postmenopausal women. Plant Foods Hum. Nutr. 2012, 67, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.; Caruso, M.C.; Guzzo, F.; Galgano, F.; Commisso, M.; Bochicchio, R.; Labella, R.; Favati, F. Nutritional quality of seeds and leaf metabolites of chia (Salvia hispanica L.) from Southern Italy. Eur. Food Res. Technol. 2015, 241, 615–625. [Google Scholar] [CrossRef]
- Da Silva, B.P.; Anunciação, P.C.; Matyelka, J.C.d.S.; Della Lucia, C.M.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Chemical composition of Brazilian chia seeds grown in different places. Food Chem. 2017, 221, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Marcinek, K.; Krejpcio, Z. Chia seeds (Salvia hispanica): Health promoting properties and therapeutic applications—A review. Roczniki Państwowego Zakładu Higieny 2017, 68, 123–129. [Google Scholar]
- Capitani, M.I.; Spotorno, V.; Nolasco, S.M.; Tomás, M.C. Physicochemical and functional characterization of by-products from chia (Salvia hispanica L.) seeds of Argentina. Food Sci. Technol. 2012, 45, 94–102. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Aliberti, L.; Amato, M.; De Feo, V.; Camele, I. Chemical composition and antimicrobial activity of chia (Salvia hispanica L.) essential oil. Eur. Food Res. Technol. 2018, 244, 1675–1682. [Google Scholar] [CrossRef]
- Marineli, R.d.S.; Moura, C.S.; Moraes, É.A.; Lenquiste, S.A.; Lollo, P.C.B.; Morato, P.N.; Amaya-Farfan, J.; Maróstica, M.R. Chia (Salvia hispanica L.) enhances HSP, PGC-1α expressions and improves glucose tolerance in diet-induced obese rats. Nutrition 2015, 31, 740–748. [Google Scholar] [CrossRef]
- Chicco, A.G.; D’Alessandro, M.E.; Hein, G.J.; Oliva, M.E.; Lombardo, Y.B. Dietary chia seed (Salvia hispanica L.) rich in a-linolenic acid improves adiposity and normalises hypertriacylglycerolaemia and insulin resistance in dyslipaemic rats. Br. J. Nutr. 2009, 101, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonte-Faria, T.; Citelli, M.; Atella, G.C.; Raposo, H.F.; Zago, L.; de Souza, T.; da Silva, S.V.; Barja-Fidalgo, C. Chia oil supplementation changes body composition and activates insulin signaling cascade in skeletal muscle tissue of obese animals. Nutrition 2019, 58, 167–174. [Google Scholar] [CrossRef]
- Da Silva, B.P.; Dias, D.M.; de Castro Moreira, M.E.; Toledo, R.C.L.; da Matta, S.L.P.; Della Lucia, C.M.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Chia seed shows good protein quality, hypoglycemic effect and improves the lipid profile and liver and intestinal morphology of wistar rats. Plant Foods Hum. Nutr. 2016, 71, 225–230. [Google Scholar] [CrossRef]
- Tavares Toscano, L.; Oliveira da Silva, C.S.; Monteiro de Almeida, A.E.; da Cruz Santos, A. Chia flour supplementation reduces blood pressure in hypertensive subjects. Plants Foods Hum. Nutr. 2014, 69, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Vuksan, V.; Whitham, D.; Sievenpiper, J.L.; Jenkins, A.L.; Rogovik, A.L.; Bazinet, R.P.; Vidgen, E.; Hanna, A. Supplementation of conventional therapy with the novel grain salba (Salvia hispanica L.) improves major and emerging cardiovascular risk factors in type 2 diabetes: Results of a randomized controlled trial. Diabetes Care 2007, 30, 2804–2810. [Google Scholar] [CrossRef] [Green Version]
- Orona-Tamayo, D.; Valverde, M.; Nieto-Rendón, B.; Paredes-Lopez, O. Inhibitory activity of chia (Salvia hispanica L.) protein fractions against angiotensin I converting enzyme and antioxidant capacity. Food Sci. Technol. 2015, 64, 236–242. [Google Scholar] [CrossRef]
- Segura-Campos, M.; Salazar-Vega, I.; Chel-Guerrero, L.; Betancur, D. Biological potential of chia (Salvia hispanica L.) protein hydrolysates and their incorporation into functional foods. LWT Food Sci. Technol. 2013, 50, 723–731. [Google Scholar] [CrossRef]
- Ho, H.; Lee, A.S.; Jovanovski, E.; Jenkins, A.L.; Desouza, R.; Vuksan, V. Effect of whole and ground Salba seeds (Salvia hispanica L.) on postprandial glycemia in healthy volunteers: A randomized controlled, dose-response trial. Eur. J. Clin. Nutr. 2013, 67, 786–788. [Google Scholar] [CrossRef] [PubMed]
- Vuksan, V.; Jenkins, A.L.; Brissette, C.; Choleva, L.; Jovanovski, E.; Gibbs, A.L.; Bazinet, R.P.; Au-Yeung, F.; Zurbau, A.; Ho, H.V.T.; et al. Salba-chia (Salvia hispanica L.) in the treatment of overweight and obese patients with type 2 diabetes: A double-blind randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Medina-Urrutia, A.; Lopez-Uribe, A.R.; El Hafidi, M.; González-Salazar, M.d.C.; Posadas-Sánchez, R.; Jorge-Galarza, E.; del Valle-Mondragón, L.; Juárez-Rojas, J.G. Chia (Salvia hispanica)-supplemented diet ameliorates non-alcoholic fatty liver disease and its metabolic abnormalities in humans. Lipids Health Dis. 2020, 19, 96. [Google Scholar] [CrossRef]
- Sargi, S.C.; Silva, B.C.; Santos, H.M.C.; Montanher, P.F.; Boeing, J.S.; Júnior, O.O.S.; Souza, N.E.; Visentainer, J.V. Antioxidant capacity and chemical composition in seeds rich in omega-3: Chia, flax, and perilla. Food Sci. 2013, 33, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Edwin, E.; Leo, M.; Segura-Campos, C.M. Neuroprotective effect from Salvia hispanica peptide fractions on pro-inflammatory modulation of HMC3 microglial cells. J. Food Biochem. 2020. [Google Scholar] [CrossRef]
- Sharma, I.; Khan, W.; Parveen, R.; Alam, M.J.; Ahmad, I.; Ansari, M.H.R.; Ahmad, S. Antiurolithiasis activity of bioactivity guided fraction of bergenia ligulata against ethylene glycol induced renal calculi in rat. Biomed. Res. Int. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Makasana, A.; Ranpariya, V.; Desai, D.; Mendpara, J.; Parekh, V. Evaluation for the anti-urolithiatic activity of Launaea procumbens against ethylene glycol-induced renal calculi in rats. Toxicol. Rep. 2014, 1, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Bawari, S.; Negi Sah, A.; Tewari, D. Antiurolithiatic activity of Daucus carota: An in vitro study. Pharmacogn. J. 2018, 10, 880–884. [Google Scholar] [CrossRef] [Green Version]
- Vertommen, J.; Van de Sompel, A.M.; Loenders, M.; Van der Velpen, C.; De Leeuw, I. Efficacy and safety of 1 month supplementation of SALBA (Salvia hispanica) grain to diet of normal adults on body parameters, blood pressure, serum lipids, minerals status and haematological parameters. Results of a pilot study. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 138–146. [Google Scholar] [CrossRef]
- Nieman, D.C.; Gillitt, N.; Jin, F.; Henson, D.A.; Kennerly, K.; Shanely, R.A.; Ore, B.; Su, M.; Schwartz, S. Chia seed supplementation and disease risk factors in overweight women: A metabolomics investigation. J. Altern. Complement. Med. 2012, 18, 700–708. [Google Scholar] [CrossRef]
- Lindsay, J.O. Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn’s disease. Gut 2006, 55, 348–355. [Google Scholar] [CrossRef]
- Kellow, N.J.; Coughlan, M.T.; Reid, C.M. Metabolic benefits of dietary prebiotics in human subjects: A systematic review of randomised controlled trials. Br. J. Nutr. 2014, 111, 1147–1161. [Google Scholar] [CrossRef]
- Pacifici, S.; Song, J.; Zhang, C.; Wang, Q.; Glahn, R.; Kolba, N.; Tako, E. Intra amniotic administration of raffinose and stachyose affects the intestinal brush border functionality and alters gut microflora populations. Nutrients 2017, 9, 304. [Google Scholar] [CrossRef]
- Yeung, C.K.; Glahn, R.E.; Welch, R.M.; Miller, D.D. Prebiotics and iron bioavailability-is there a connection? J. Food Sci. 2005, 70, 88–92. [Google Scholar] [CrossRef]
- Miśta, D.; Króliczewska, B.; Pecka-Kiełb, E.; Kapuśniak, V.; Zawadzki, W.; Graczyk, S.; Kowalczyk, A.; Łukaszewicz, E.; Bednarczyk, M. Effect of in ovo injected prebiotics and synbiotics on the caecal fermentation and intestinal morphology of broiler chickens. Anim. Prod. Sci. 2017, 57, 1884. [Google Scholar] [CrossRef]
- Sarao, L.K.; Arora, M. Probiotics, prebiotics, and microencapsulation: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 344–371. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Conesa, D.; López, G.; Ros, G. Effect of probiotic, prebiotic and synbiotic follow-up infant formulas on iron bioavailability in rats. Food Sci. Technol. Int. 2007, 13, 69–77. [Google Scholar] [CrossRef]
- Weinborn, V.; Valenzuela, C.; Olivares, M.; Arredondo, M.; Weill, R.; Pizarro, F. Prebiotics increase heme iron bioavailability and do not affect non-heme iron bioavailability in humans. Food Funct. 2017, 8, 1994–1999. [Google Scholar] [CrossRef]
- Przybylska-Balcerek, A.; Stuper-Szablewska, K. Phenolic acids used in the cosmetics industry as natural antioxidants. Eur. J. Med. Technol. 2019, 4, 24–32. [Google Scholar]
- Vegan Cosmetics—Definition. Available online: https://biotechnologia.pl/kosmetologia/weganizm-nie-tylko-w-diecie-czyli-co-wiemy-o-kosmetykach-weganskich,16677#:~:text=Kosmetykiwega%C5%84skiebywaj%C4%85cz%C4%99stookre%C5%9Blane,eksperymentaminazwierz%C4%99tach%20%5B1%5D (accessed on 11 October 2021).
- Natural Cosmetics—Definition. Available online: http://biotechnologia.pl/kosmetologia/artykuly/kosmetyki-naturalne,10830 (accessed on 11 October 2021).
- Jeong, S.K.; Park, H.J.; Park, B.D.; Kim, I.-H. Effectiveness of topical chia seed oil on pruritus of end-stage renal disease (ESRD) patients and healthy volunteers. Ann. Dermatol. 2010, 22, 143. [Google Scholar] [CrossRef] [Green Version]
- Opinion on the safety of ‘Chia seeds (Salvia hispanica L.) and ground whole chia seeds’ as a food ingredient. EFSA J. 2009, 7, 996. [CrossRef]
- Borneo, R.; Aguirre, A.; Leon, A.E. Chia (Salvia hispanica L.) gel can be used as egg or oil replacer in cake formulations. J. Am. Diet. Assoc. 2010, 110, 946–949. [Google Scholar] [CrossRef]
- Dos Reis Gallo, L.R.; Botelho, R.B.A.; Ginani, V.C.; de Lacerda de Oliveira, L.; Riquette, R.F.R.; dos Santos Leandro, E. Chia (Salvia hispanica L.) gel as egg replacer in chocolate cakes: Applicability and microbial and sensory qualities after storage. J. Culin. Sci. Technol. 2020, 18, 29–39. [Google Scholar] [CrossRef]
- Felisberto, M.H.F.; Wahanik, A.L.; Gomes-Ruffi, C.R.; Clerici, M.T.P.S.; Chang, Y.K.; Steel, C.J. Use of chia (Salvia hispanica L.) mucilage gel to reduce fat in pound cakes. LWT Food Sci. Technol. 2015, 63, 1049–1055. [Google Scholar] [CrossRef] [Green Version]
- Zi, C.T.; Yang, L.; Xu, F.Q.; Dong, F.W.; Yang, D.; Li, Y.; Ding, Z.T.; Zhou, J.; Jiang, Z.H.; Hu, J.M. Synthesis and anticancer activity of dimeric podophyllotoxin derivatives. Drug Des. Dev. Ther. 2018, 12, 3393–3406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Lin, H.-W.; Lin, Y.-L.; Yang, D.-J.; Yu, Y.-S.; Chen, J.-W.; Wang, S.-Y.; Chen, Y.-C. Nutritional composition in the chia seed and its processing properties on restructured ham-like products. J. Food Drug Anal. 2018, 26, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, G.; Herranz Barbero, A.; Borrás-Novell, C.; Alsina Casanova, M.; Aldecoa-Bilbao, V.; Andreu-Fernández, V.; Pascual Tutusaus, M.; Ferrero Martínez, S.; Gómez Roig, M.; García-Algar, O. The effects of vegetarian and vegan diet during pregnancy on the health of mothers and offspring. Nutrients 2019, 11, 557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-López, J.; Viuda-Martos, M.; Sayas-Barberá, M.E.; Navarro-Rodríguez de Vera, C.; Lucas-González, R.; Roldán-Verdú, A.; Botella-Martínez, C.; Pérez-Alvarez, J.A. Chia, quinoa, and their coproducts as potential antioxidants for the meat industry. Plants 2020, 9, 1359. [Google Scholar] [CrossRef]
- Carvalho, C.; Católica, U.; Bosco, D.; Regina, C.; Arfux, B.; Menegati, C.D.F.; Moreira, R. Cultivo in vitro de Salvia hispanica. Rev. Eletrônica Gestão 2015, 19, 1555–1560. [Google Scholar]
- Zayova, E.; Nikolova, M.; Dimitrova, L.; Petrova, M. Comparative study of in vitro, ex vitro and in vivo propagated Salvia hispanica (chia) plants: Morphometric analysis and antioxidant activity. AgroLife Sci. J. 2016, 5, 166–174. [Google Scholar]
- Yadav, A.; Kothari, S.L.; Kachhwaha, S.; Joshi, A. In vitro propagation of chia (Salvia hispanica L.) and assessment of genetic fidelity using random amplified polymorphic DNA and intersimple sequence repeat molecular markers. J. Appl. Biol. Biotechnol. 2019, 7, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Marconi, P.L.; López, M.C.; De Meester, J.; Bovjin, C.; Alvarez, M.A. In vitro establishment of Salvia hispanica L. plants and callus. Biotecnol. Veg. 2013, 4, 203–207. [Google Scholar]
- Bueno, M.; Di Sapio, O.; Barolo, M.; Villalonga, M.E. In vitro response of different Salvia hispanica L. (Lamiaceae) explants. Mol. Med. Chem. 2010, 21, 125–126. [Google Scholar]

| Unsaturated Fatty Acids | Origin of Chia Seeds | References | ||
|---|---|---|---|---|
| α-Linolenic Acid (ALA) n-3 | Linoleic Acid (LA) n-6 | Oleic Acid n-9 | ||
| (C18H30O2) | (C18H32O2) | (C18H34O2) | ||
| 61.9 | 19.9 | 7.5 | West Mexico (Sinaloa) | [63] |
| 63.4 | 19.8 | 8.2 | Central West Mexico (Jalisco) | [63] |
| 62.02 | 17.36 | 10.55 | Southeast Brazil (Jacareia) | [62] |
| 64.6 | 18.6 | 6.8 | Argentina | [64] |
| 61.92 | 18.99 | 6.77 | France | [39] |
| 60.94 | 19.16 | 6.87 | France | [39] |
| Chlorogenic Acid | Ferulic Acid | Gallic Acid | Caffeic Acid | p-Coumaric Acid | Rosmarinic Acid | References |
|---|---|---|---|---|---|---|
| nd | t | 1.15 | 2.74 | nd | 92.67 | [72] |
| nd | 3.587 | 4.256 | 12.536 | 2.596 | 65.398 | [73] |
| 4.59–10.20 | nd | nd | 0.30–0.68 | nd | nd | [66] |
| nd | nd | 0.005 | - | 0.024 | nd | [74] |
| 0.468 | nd | nd | 3.089 | nd | nd | [62] |
| nt | nt | nd | nt | nd | nt | [75] |
| Macroelements | References | |||||||
|---|---|---|---|---|---|---|---|---|
| Phosphorus (P) | Calcium (Ca) | Potassium (K) | Magnesium (Mg) | Sodium (Na) | Iron (Fe) | Manganese (Mn) | Sulfur (S) | |
| 860 | 631 | 407 | 335 | 16 | 7.72 | 2.72 | nt | [47] |
| 530–640 | 430–480 | 550–620 | 330–350 | 140–150 | 7.69–9.39 | 2.48–4.05 | 150–200 | [76] |
| 696–799 | 580–624 | 666–870 | 369–403 | nt | 10.9–24.4 | nt | nt | [77] |
| 901.25–1247.60 | 561.50–806.00 | nt | 322.00–462.40 | nt | 11.73–14.27 | nt | nt | [78] |
| 919 | 456 | 726 | 449 | 0.26 | 9.18 | 3.79 | nt | [74] |
| Microelements | ||||||||
| Zinc (Zn) | Copper (Cu) | Molybdenum (Mo) | Selenium (Se) | |||||
| 4.58 | 0.92 | 0.20 | 0.06 | [47] | ||||
| 3.65–3.76 | 0.63–1.32 | nt | nt | [76] | ||||
| 6.0–6.9 | nt | nt | 0.08 | [77] | ||||
| 0.60–10.00 | 1.87–2.42 | nt | nt | [78] | ||||
| 6.47 | 1.86 | nt | nt | [74] | ||||
| Vitamins | Content (mg per 100 g of dry seeds) | |
|---|---|---|
| acc. to USDA (National Nutrient Database for Standard Reference) [71] | acc. to Knez et al. [9] | |
| Niacin (vitamin B3) | 8.80 mg | 8.83 mg |
| Ascorbic acid (vitamin C) | 1.60 mg | 1.60 mg |
| Thiamine (vitamin B1) | 0.60 mg | 0.62 mg |
| Vitamin E | 0.50 mg | 0.50 mg |
| Riboflavin (vitamin B2) | 0.20 mg | 0.17 mg |
| Vitamin A | 54 µg | 54 µg |
| Profile of Action | Raw Material | Mechanism | References |
|---|---|---|---|
| Lipid-lowering, hypoglycemic | Chia seed oil, chia seeds | Prevention of metabolic diseases by lowering TGs, TC, LDL, and VLDL and increasing HDL (by inhibiting 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase), blocking the mevalonate metabolic pathway, lowering ALT and ASP, improving liver function, and improving postprandial glucose levels | [33,34,66,93] |
| Hypotensive | Chia seeds, chia seed flour, chia seed oil | Prevention of arterial hypertension by lowering systolic blood pressure | [82,84,96,97] |
| Weight-reducing | Chia seeds | Preventing overweight and obesity by inhibiting adipogenesis and reducing the level of PPAR-γ protein | [34,85] |
| Improving the function of the digestive tract | Chia seeds | Prevention of diseases of the gastrointestinal tract and intestinal dysbiosis by improving the absorption of nutrients (especially Fe and Zn), intensifying fermentation processes in the intestines, and increasing the production of short-chain fatty acids by intestinal bacteria, surface of intestinal villi, proliferation of enterocytes, and increase of beneficial intestinal bacteria, mainly Bifidobacterium and Lactobacillus in the cecum | [58] |
| Neuroprotective and anti-inflammatory | Chia seed oil | Prevention of neurodegenerative diseases by protective effect on microglial cells (HMC3) and reducing levels of inflammatory mediators (TNF-α, IL-6, NO, and H2O2) and ROS. | [33,92] |
| Preventing kidney stones | Chia seed methanol extract | Prevention of kidney stones by lowering the levels of creatinine, urea, and uric acid in the blood serum, inhibition of the initial stages of CaOx crystal formation, including nucleation, aggregation, and growth phases | [36,93,94,95] |
| Hepatoprotective | Chia seeds | Preventing liver disease by reducing the amount of intrahepatic fat, lipid deposition in hepatocytes, and increasing in plasma activity of antioxidant enzymes: catalase (CAT), glutathione peroxidase (GPx), and glutathione (GSH) | [67,90] |
| Antioxidant | Chia seeds | Preventing inflammation by reducing the level of CRP and inflammatory markers (tumor necrosis factor-α, nitric oxide, hydrogen peroxide, interleukin-6), antiradical activity, and the ability to inhibit lipoxygenase-5 and cyclooxygenases 1 and 2 | [47,68,69] |
| Name according to CosIng | Application |
|---|---|
| S. hispanica seed | Abrasive, scrubbing agent |
| S. hispanica seed powder | Abrasive, scrubbing agent |
| S. hispanica seed oil | Moisturizing agent, nourishing effect on the epidermis |
| S. hispanica seed extract | Emollient, nourishing effect on the epidermis |
| S. hispanica herb oil | For the production of perfumes and aromas, functional fragrances |
| S. hispanica herb extract | For the production of perfumes and aromas, functional fragrances |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motyka, S.; Koc, K.; Ekiert, H.; Blicharska, E.; Czarnek, K.; Szopa, A. The Current State of Knowledge on Salvia hispanica and Salviae hispanicae semen (Chia Seeds). Molecules 2022, 27, 1207. https://doi.org/10.3390/molecules27041207
Motyka S, Koc K, Ekiert H, Blicharska E, Czarnek K, Szopa A. The Current State of Knowledge on Salvia hispanica and Salviae hispanicae semen (Chia Seeds). Molecules. 2022; 27(4):1207. https://doi.org/10.3390/molecules27041207
Chicago/Turabian StyleMotyka, Sara, Katarzyna Koc, Halina Ekiert, Eliza Blicharska, Katarzyna Czarnek, and Agnieszka Szopa. 2022. "The Current State of Knowledge on Salvia hispanica and Salviae hispanicae semen (Chia Seeds)" Molecules 27, no. 4: 1207. https://doi.org/10.3390/molecules27041207
APA StyleMotyka, S., Koc, K., Ekiert, H., Blicharska, E., Czarnek, K., & Szopa, A. (2022). The Current State of Knowledge on Salvia hispanica and Salviae hispanicae semen (Chia Seeds). Molecules, 27(4), 1207. https://doi.org/10.3390/molecules27041207