Chemoselective Preparation of New Families of Phenolic-Organoselenium Hybrids—A Biological Assessment
Abstract
:1. Introduction
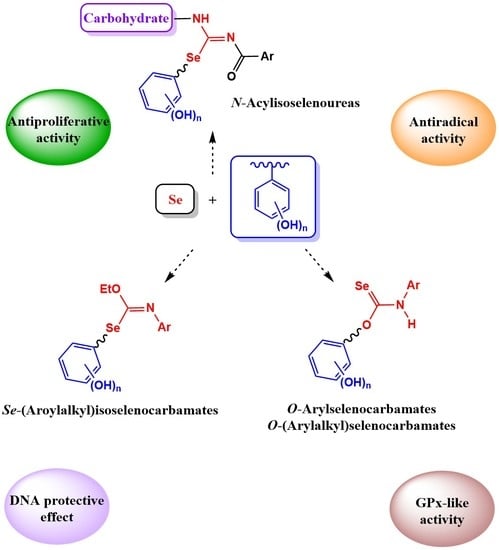
2. Results and Discussion
2.1. Chemistry
2.2. Biological Assays
2.2.1. Antioxidant Properties
DPPH Assay
DNA Oxidation Inhibition
Glutathione Peroxidase-like Activity
2.2.2. Antiproliferative Activity
3. Materials and Methods
3.1. General Procedures
3.2. Antioxidant Assays
3.2.1. Free Radical-Induced DNA Degradation Inhibition
3.2.2. Glutathione Peroxidase Mimicry
3.2.3. Statistical Analysis
3.3. Chemistry
3.3.1. General Procedure for the Synthesis of Se-Phenacyl Isoselenoureas 7–9
3.3.2. General Procedure for the Preparation of Se-Phenacyl Isoselenocarbamates 16–21
3.3.3. General Procedure for the Preparation of Selenocarbamates 24, 25
3.3.4. General Procedure for the Preparation of Selenocarbamates 26, 27
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jain, V.K. An Overview of organoselenium chemistry: From fundamentals to synthesis. In Organoselenium Compounds in Biology and Medicine: Synthesis, Biological and Therapeutic Treatments; Jain, V.K., Priyadarsini, K.I., Eds.; RSC: London, UK, 2017; pp. 1–33. [Google Scholar]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwaoka, M.; Arai, K. From sulfur to selenium. A new research arena in chemical biology and biological chemistry. Curr. Chem. Biol. 2013, 7, 2–24. [Google Scholar] [CrossRef]
- López, Ó.; Merino-Montiel, P.; Fernández-Bolaños, J.G. Synthesis of organoselenium derivatives of biological relevance. In Selenium: Chemistry, Analysis, Function and Effects; Preedy, V.R., Ed.; RSC: London, UK, 2015; pp. 40–64. [Google Scholar]
- Arora, A.; Singh, S.; Oswal, P.; Nautiyal, D.; Rao, G.K.; Kumar, S.; Kumar, A. Preformed molecular complexes of metals with organoselenium ligands: Syntheses and applications in catalysis. Coord. Chem. Rev. 2021, 438, 213885. [Google Scholar] [CrossRef]
- Shao, L.; Li, Y.; Lu, J.; Jiang, X. Recent progress in selenium-catalyzed organic reactions. Org. Chem. Front. 2019, 6, 2999–3041. [Google Scholar] [CrossRef]
- Singh, F.V.; Wirth, T. Selenium reagents as catalysts. Catal. Sci. Technol. 2019, 9, 1073–1091. [Google Scholar] [CrossRef]
- Senatore, R.; Castoldi, L.; Ielo, L.; Holzer, W.; Pace, V. Expeditious and chemoselective synthesis of α-aryl and α-alkyl selenomethylketones via homologation chemistry. Org. Lett. 2018, 20, 2685–2688. [Google Scholar] [CrossRef]
- Zheng, G.; Zhao, J.; Li, Z.; Zhang, Q.; Sun, J.; Sun, H.; Zhang, Q. Highly regio- and stereoselective intermolecular seleno- and thioamination of alkynes. Chem. Eur. J. 2016, 22, 3513–3518. [Google Scholar] [CrossRef]
- Tao, Z.; Gilbert, B.B.; Denmark, S.E. Catalytic, enantioselective syn-diamination of alkenes. J. Am. Chem. Soc. 2019, 141, 19161–19170. [Google Scholar] [CrossRef]
- Kostić, M.D.; Divac, M.V. Green solvents in organoselenium chemistry. Environ. Chem. Lett. 2019, 17, 897–915. [Google Scholar] [CrossRef]
- Mukherjee, A.J.; Zade, S.S.; Singh, H.B.; Sunoj, R.B. Organoselenium chemistry: Role of intramolecular interactions. Chem. Rev. 2010, 110, 4357–4416. [Google Scholar] [CrossRef]
- Fourmigue, M.; Dhaka, A. Chalcogen bonding in crystalline diselenides and selenocyanates: From molecules of pharmaceutical interest to conducting materials. Coord. Chem. Rev. 2020, 403, 213084. [Google Scholar] [CrossRef]
- Young, C.M.; Elmi, A.; Pascoe, D.J.; Morris, R.K.; McLaughlin, C.; Woods, A.M.; Frost, A.B.; de la Houpliere, A.; Ling, K.B.; Smith, T.K.; et al. The importance of 1,5-oxygen⋅chalcogen interactions in enantioselective isochalcogenourea catalysis. Angew. Chem. Int. Ed. 2020, 59, 3705–3710. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, D.J.; Ling, K.B.; Cockroft, S.L. The origin of chalcogen-bonding interactions. J. Am. Chem. Soc. 2017, 139, 15160–15167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casula, A.; Begines, P.; Bettoschi, A.; Fernandez-Bolaños, J.G.; Isaia, F.; Lippolis, V.; López, Ó.; Picci, G.; Scorciapino, M.A.; Caltagirone, C. Selenoureas for anion binding as molecular logic gates. Chem. Commun. 2017, 53, 11869–11872. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.; Yang, S.; Huang, H.; Zong, H.; Song, L.; Fan, H.; Sun, X. Chirality sensing of tertiary alcohols by a novel strong hydrogen-bonding donor—Selenourea. Chem. Sci. 2016, 7, 932–938. [Google Scholar] [CrossRef] [Green Version]
- Huynh, H.-T.; Jeannin, O.; Fourmigué, M. Organic selenocyanates as strong and directional chalcogen bond donors for crystal engineering. Chem. Commun. 2017, 53, 8467–8469. [Google Scholar] [CrossRef]
- Bhabak, K.P.; Mugesh, G. Functional mimics of glutathione peroxidase: Bioinspired synthetic antioxidants. Acc. Chem. Res. 2010, 43, 1408–1419. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Galant, L.S.; Rafique, J.; Braga, A.L.; Braga, F.C.; Saba, S.; Radi, R.; da Rocha, J.B.T.; Santi, C.; Monsalve, M.; Farina, M.; et al. The thiol-modifier effects of organoselenium compounds and their cytoprotective actions in neuronal cells. Neurochem. Res. 2021, 46, 120–130. [Google Scholar] [CrossRef]
- Zhang, D.-W.; Yan, H.-L.; Xu, X.-S.; Xu, L.; Yin, Z.-H.; Chang, S.; Luo, H. The selenium-containing drug ebselen potently disrupts LEDGF/p75-HIV-1 integrase interaction by targeting LEDGF/p75. J. Enzyme Inhib. Med. Chem. 2020, 35, 906–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasim, M.J.; Witek, K.; Kincses, A.; Abdin, A.Y.; Żesławska, E.; Marć, M.A.; Gajdács, M.; Spengler, G.; Nitek, W.; Latacz, G.; et al. Pronounced activity of aromatic selenocyanates against multidrug resistant ESKAPE bacteria. New J. Chem. 2019, 43, 6021–6031. [Google Scholar] [CrossRef] [Green Version]
- Al-Tamimi, A.-M.S.; Etxebeste-Mitxeltorena, M.; Sanmartín, C.; Jiménez-Ruiz, A.; Syrjänen, L.; Parkkila, S.; Selleri, S.; Carta, F.; Angeli, A.; Supuran, C.T. Discovery of new organoselenium compounds as antileishmanial agents. Bioorg. Chem. 2019, 86, 339–345. [Google Scholar] [CrossRef]
- Birmann, P.T.; Sousa, F.S.S.; de Oliveira, D.H.; Domingues, M.; Vieira, B.M.; Lenardão, E.J.; Savegnago, L. 3-(4-Chlorophenylselanyl)-1-methyl-1H-indole, a new selenium compound elicits an antinociceptive and anti-inflammatory effect in mice. Eur. J. Pharmacol. 2018, 827, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Zamberlan, D.C.; Arantes, L.P.; Machado, M.L.; Golombieski, R.; Soares, F.A.A. Diphenyl-diselenide suppresses amyloid-β peptide in Caenorhabditis elegans model of Alzheimer’s disease. Neuroscience 2014, 278, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Angeli, A.; Abbas, G.; Del Prete, S.; Carta, F.; Capasso, C.; Supuran, C.T. Acyl selenoureido benzensulfonamides show potent inhibitory activity against carbonic anhydrases from the pathogenic bacterium Vibrio cholerae. Bioorg. Chem. 2017, 75, 170–172. [Google Scholar] [CrossRef]
- Chen, Z.; Lai, H.; Hou, L.; Chen, T. Rational design and action mechanisms of chemically innovative organoselenium in cancer therapy. Chem. Commun. 2020, 56, 179–196. [Google Scholar] [CrossRef]
- Gandin, V.; Khalkar, P.; Braude, J.; Fernandes, A.P. Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Radic. Biol. Med. 2018, 127, 80–97. [Google Scholar] [CrossRef]
- Berntssen, M.H.G.; Sundal, T.K.; Olsvik, P.A.; Amlund, H.; Rasinger, J.D.; Sele, V.; Hamre, K.; Hillestad, M.; Buttle, L.; Ørnsrud, R. Sensitivity and toxic mode of action of dietary organic and inorganic selenium in Atlantic salmon (Salmo salar). Aquat. Toxicol. 2017, 192, 116–126. [Google Scholar] [CrossRef]
- Frieben, E.E.; Amin, S.; Sharma, A.K. Development of isoselenocyanate compounds’ syntheses and biological applications. J. Med. Chem. 2019, 62, 5261–5275. [Google Scholar] [CrossRef]
- Begines, P.; Sevilla-Horrillo, L.; Puerta, A.; Puckett, R.; Bayort, S.; Lagunes, I.; Maya, I.; Padrón, J.M.; López, Ó.; Fernández-Bolaños, J.G. Masked phenolic-selenium conjugates: Potent and selective antiproliferative agents overcoming P-gp resistance. Pharmaceuticals 2020, 13, 358. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Aguilar, A.; Romero-Hernández, L.L.; Arenas-González, A.; Merino-Montiel, P.; Montiel-Smith, S.; Meza-Reyes, S.; Vega-Báez, J.L.; Plata, G.B.; Padrón, J.M.; López, Ó.; et al. New selenosteroids as antiproliferative agents. Org. Biomol. Chem. 2017, 15, 5041–5054. [Google Scholar] [CrossRef] [PubMed]
- Plano, D.; Karelia, D.N.; Pandey, M.K.; Spallholz, J.E.; Amin, S.; Sharma, A.K. Design, Synthesis, and biological evaluation of novel selenium (Se-NSAID) molecules as anticancer agents. J. Med. Chem. 2016, 59, 1946–1959. [Google Scholar] [CrossRef]
- Roldán-Peña, J.R.; Alejandre-Ramos, D.; López, Ó.; Maya, I.; Lagunes, I.; Padrón, J.M.; Peña-Altamira, L.E.; Bartolini, M.; Monti, B.; Bolognesi, M.L.; et al. New tacrine dimers with antioxidant linkers as dual drugs: Anti-Alzheimer’s and antiproliferative agents. Eur. J. Med. Chem. 2017, 138, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz, E.H.G.; Silvers, M.A.; Jardim, G.A.M.; Resende, J.M.; Cavalcanti, B.C.; Bomfim, I.S.; Pessoa, C.; de Simone, C.A.; Botteselle, G.V.; Braga, A.L.; et al. Synthesis and antitumor activity of selenium-containing quinone-based triazoles possessing two redox centres, and their mechanistic insights. Eur. J. Med. Chem. 2016, 122, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagunes, I.; Begines, P.; Silva, A.; Galán, A.R.; Puerta, A.; Fernandes, M.X.; Maya, I.; Fernández-Bolaños, J.G.; López, Ó.; Padrón, J.M. Selenocoumarins as new multitarget antiproliferative agents: Synthesis, biological evaluation and in silico calculations. Eur. J. Med. Chem. 2019, 179, 493–501. [Google Scholar] [CrossRef]
- Begines, P.; Oliete, A.; López, Ó.; Maya, I.; Plata, G.B.; Padrón, J.M.; Fernández-Bolaños, J.G. Chalcogen-containing phenolics as antiproliferative agents. Future Med. Chem. 2018, 10, 319–334. [Google Scholar] [CrossRef]
- Alcolea, V.; Plano, D.; Encío, I.; Palop, J.A.; Sharma, A.K.; Sanmartín, C. Chalcogen containing heterocyclic scaffolds: New hybrids with antitumoral activity. Eur. J. Med. Chem. 2016, 123, 407–418. [Google Scholar] [CrossRef]
- Maiyo, F.; Singh, M. Selenium nanoparticles: Potential in cancer gene and drug delivery. Nanomedicine 2017, 12, 1075–1089. [Google Scholar] [CrossRef]
- Patra, A.R.; Roy, S.S.; Basu, A.; Bhuniya, A.; Bhattacharjee, A.; Hajra, S.; Sk, U.H.; Baral, R.; Bhattacharya, S. Design and synthesis of coumarin-based organoselenium as a new hit for myeloprotection and synergistic therapeutic efficacy in adjuvant therapy. Sci. Rep. 2018, 8, 2194. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, H.; Qi, R.; Tsao, R.; Mine, Y. Recent advances in the understanding of the health benefits and molecular mechanisms associated with green tea polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef]
- Fernández-Bolaños, J.G.; López, Ó.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Hydroxytyrosol and derivatives: Isolation, synthesis, and biological properties. Curr. Org. Chem. 2008, 12, 442–463. [Google Scholar] [CrossRef]
- Franconetti, A.; López, Ó.; Fernández-Bolaños, J.G. Carbohydrates: Potential sweet tools against cancer. Curr. Med. Chem. 2020, 27, 1206–1242. [Google Scholar] [CrossRef]
- Garnica, P.; Encío, I.; Plano, D.; Palop, J.A.; Sanmartin, C. Combined acylselenourea-diselenide structures: New potent and selective antitumoral agents as autophagy activators. ACS Med. Chem. Lett. 2018, 9, 306–311. [Google Scholar] [CrossRef]
- Babiano Caballero, R.; Fuentes Mota, J.; Galbis Pérez, J.A. A new method for the preparation of acylated glycosylamines and their transformations into glycosyl isothiocyanates and N, N′-diglycosylthioureas. Carbohydr. Res. 1986, 154, 280–288. [Google Scholar] [CrossRef]
- Douglass, I.B. Acylselenoureas. J. Am. Chem. Soc. 1937, 59, 740–742. [Google Scholar] [CrossRef]
- López, Ó.; Maza, S.; Ulgar, V.; Maya, I.; Fernández-Bolaños, J.G. Synthesis of sugar-derived isoselenocyanates, selenoureas, and selenazoles. Tetrahedron 2009, 65, 2556–2566. [Google Scholar] [CrossRef]
- Ho, T.L. Hard soft acids bases (HSAB) principle and organic chemistry. Chem. Rev. 1975, 75, 1–20. [Google Scholar] [CrossRef]
- Ach, D.; Reboul, V.; Metzner, P. Benzylic metallation of thiobenzamides and thionaphthamides. Eur. J. Org. Chem. 2002, 2002, 2573–2586. [Google Scholar] [CrossRef]
- Sambrook, M.R.; Beer, P.D.; Wisner, J.A.; Paul, R.L.; Cowley, A.R.; Szemes, F.; Drew, M.G.B. Anion-templated assembly of pseudorotaxanes: Importance of anion template, strength of ion-pair thread association, and macrocycle ring size. J. Am. Chem. Soc. 2005, 127, 2292–2302. [Google Scholar] [CrossRef]
- Wu, R.; Odom, J.D.; Dunlap, R.B.; Silks, L.A., III. Reaction of alcohols (via the Mitsunobu reaction) and alkyl halides with chiral selone derivatizing agents. Tetrahedron Asymmetry 1999, 10, 1465–1470. [Google Scholar] [CrossRef]
- Merino-Montiel, P.; Maza, S.; Martos, S.; López, Ó.; Maya, I.; Fernández-Bolaños, J.G. Synthesis and antioxidant activity of O-alkyl selenocarbamates, selenoureas and selenohydantoins. Eur. J. Pharm. Sci. 2013, 48, 582–592. [Google Scholar] [CrossRef]
- Asanuma, Y.; Fujiwara, S.-i.; Shin-ike, T.; Kambe, N. Selenoimidoylation of alcohols with selenium and isocyanides and its application to the synthesis of selenium-containing heterocycles. J. Org. Chem. 2004, 69, 4845–4848. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Parekh, S.I.; Tajbakhsh, M.; Theodorakis, E.A.; Tse, C.L. A convenient and high yielding procedure for the preparation of isoselenocyanates. Synthesis and reactivity of O-alkylselenocarbamates. Tetrahedron 1994, 50, 639–654. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Gredilla, R.; Bohr, V.A.; Stevnsner, T. Mitochondrial DNA repair and association with aging—An update. Exp. Geront. 2010, 45, 478–488. [Google Scholar] [CrossRef] [Green Version]
- Mao, P.; Reddy, P.H. Aging and amyloid beta-induced oxidative DNA damage and mitochondrial dysfunction in Alzheimer’s disease: Implications for early intervention and therapeutics. Biochim. Biophys. Acta 2011, 1812, 1359–1370. [Google Scholar] [CrossRef] [Green Version]
- Scott, T.L.; Rangaswamy, S.; Wicker, C.A.; Izumi, T. Repair of oxidative DNA damage and cancer: Recent progress in DNA base excision repair. Antioxid. Redox Signal. 2014, 20, 708–726. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Liu, Z.Q. The protective effect of hydroxyl-substituted Schiff bases on the radical-induced oxidation of DNA. J. Phys. Org. Chem. 2009, 22, 791–798. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef]
- Nascimento, V.; Alberto, E.E.; Tondo, D.W.; Dambrowski, D.; Detty, M.R.; Nome, F.; Braga, A.L. GPx-Like activity of selenides and selenoxides: Experimental evidence for the involvement of hydroxy perhydroxy selenane as the active species. J. Am. Chem. Soc. 2012, 134, 138–141. [Google Scholar] [CrossRef]
- Merino-Montiel, P.; López, Ó.; Fernández-Bolaños, J.G. l-Isofucoselenofagomine and derivatives: Dual activities as antioxidants and as glycosidase inhibitors. Tetrahedron 2012, 68, 3591–3595. [Google Scholar] [CrossRef]
- Selvakumar, K.; Shah, P.; Singh, H.B.; Butcher, R.J. Selenazolyl-hydrazones as novel selective MAO inhibitors with antiproliferative and antioxidant activities: Experimental and in-silico studies Synthesis, structure, and glutathione peroxidase-like activity of amino acid containing ebselen analogues and diaryl diselenides. Chem. Eur. J. 2011, 17, 12741–12755. [Google Scholar]
- Iwaoka, M.; Kumakura, F. Applications of water-soluble selenides and selenoxides to protein chemistry. Phosphorous Sulphur Silicon Relat. Elem. 2008, 183, 1009–1017. [Google Scholar] [CrossRef]
- Elshaflu, H.; Todorović, T.R.; Nikolić, M.; Lolić, A.; Višnjevac, A.; Hagenow, S.; Padrón, J.M.; García-Sosa, A.T.; Djordjević, L.S.; Grubišić, S.; et al. Selenazolyl-hydrazones as novel selective MAO inhibitors with antiproliferative and antioxidant activities: Experimental and in-silico studies. Front. Chem. 2018, 6, 247. [Google Scholar] [CrossRef] [Green Version]





| Compound | DPPH (EC50, µM) c | %DNA Oxidation Inhibition a,c | |
|---|---|---|---|
| Selenourea | 3 | 22 ± 1 | 9.2 ± 3.5 |
| Isoselenoureas | 7 | >250 | 8.2 ± 2.2 |
| 8 | >250 | 3.8 ± 5.6 | |
| 9 | 15 ± 1 | 32 ± 2 | |
| O-Phenacyl isoselenocarbamates | 16 | >250 | 1.9 ± 0.20 |
| 17 | >250 | 6.5 ± 3.9 | |
| 18 | 8.0 ± 0.2 | 38 ± 5 | |
| 19 | >250 | 9.1 ± 0.20 | |
| 20 | >250 | 1.3 ± 1.7 | |
| 21 | 7.8 ± 0.3 | 31 ± 5 | |
| Selenocarbamates | 24 | 64 ± 2.0 | 9.4 ± 5.0 |
| 25a | 12 ± 1 | 33 ± 1 | |
| 25b | 16 ± 3 | N.T. b | |
| 25c | 6.3 ± 0.8 | N.T.b | |
| 25d | 4.7 ± 0.5 | N.T.b | |
| 25e | 4.8 ± 0.3 | N.T.b | |
| 25f | 5.2 ± 0.2 | N.T.b | |
| 26 | 71 ± 1 | 29 ± 9 | |
| 27 | 15 ± 1 | 32 ± 1 | |
| Controls | 22 (Tyr) | >500 | 18 ± 1 |
| 23 (HTyr) | 15 ± 2 | 41 ± 3 | |
| BHT [45] | 70 ± 9 | 4.1 ± 1.0 | |
| Compound b | t1/2 (min) | |
|---|---|---|
| Selenourea | 3 | 5.9 |
| Isoselenoureas | 7 | 3.4 |
| 8 | 5.9 | |
| 9 | No activity | |
| O-Phenacyl isoselenocarbamates | 16 | 233 |
| 17 | 7.1 | |
| 18 | 2.8 | |
| 19 | No activity | |
| 20 | 4.9 | |
| 21 | 3.4 | |
| Selenocarbamates | 24 | <0.5 5.0 c |
| 25a | 1.0 8.7 c | |
| 26 | 2.9 18.3 c | |
| 27 | 0.9 9.4 c | |
| Control | HTyr | No activity |
| Compound | A549 (Lung) | HBL-100 (Breast) | Hela (Cervix) | SW1573 (Lung) | T-47D (Breast) | WiDr (Colon) | |
|---|---|---|---|---|---|---|---|
| Selenourea | 3 | 3.2 ± 0.84 | 3.5 ± 1.2 | 3.3 ± 1.4 | 4.4 ± 1.5 | 3.3 ± 0.20 | 4.9 ± 1.4 |
| Isoselenoureas | 8 | 36 ± 3.3 | 39 ± 6.6 | 42 ± 9.5 | 35 ± 1.4 | 29 ± 5.6 | 45 ± 16 |
| 9 | >100 | >100 | >100 | >100 | >100 | >100 | |
| Isoselenocarbamates | 16 | 8.6 ± 3.5 | 26 ± 3.2 | 31 ± 3.1 | 31 ± 5.9 | 24 ± 4.4 | 45 ± 20 |
| 17 | 6.9 ± 1.3 | 10 ± 0.39 | 14 ± 0.66 | 18 ± 4.8 | 8.8 ± 3.7 | 20 ± 6.2 | |
| 19 | 24 ± 6.1 | 30 ± 3.5 | 20 ± 5.5 | 90 ± 17 | 84 ± 11 | 34 ± 6.1 | |
| 20 | 19 ± 0.32 | 19 ± 4.2 | 15 ± 2.0 | 17 ± 3.5 | 18 ± 2.3 | 21 ± 2.9 | |
| 21 | 16 ± 0.62 | 16 ± 0.51 | 13 ± 1.6 | 14 ± 2.6 | 16 ± 1.0 | 18 ± 2.0 | |
| Selenocarbamates | 24 | 13 ± 0.52 | 20 ± 1.8 | 15 ± 2.3 | 18 ± 5.1 | 23 ± 9.2 | 25 ± 6.1 |
| 25b | 7.5 ± 1.5 | 18 ± 0.21 | 19 ± 1.1 | 12 ± 1.7 | 17 ± 1.3 | 18 ± 1.2 | |
| 25c | 4.5 ± 1.8 | 16 ± 2.6 | 13 ± 2.5 | 5.9 ± 1.5 | 20 ± 4.9 | 20 ± 7.0 | |
| 25d | 8.5 ± 4.4 | 18 ± 2.7 | 15 ± 3.6 | 6.5 ± 2.9 | 21 ± 10 | 25 ± 11 | |
| 25e | 9.0 ± 3.8 | 18 ± 3.1 | 14 ± 4.7 | 6.6 ± 1.9 | 23 ± 8.6 | 20 ± 5.5 | |
| 25f | 11 ± 4.0 | 12 ± 2.7 | 10 ± 2.7 | 3.7 ± 1.3 | 16 ± 4.5 | 11 ± 2.0 | |
| 26 | 14 ± 1.8 | 18 ± 3.3 | 14 ± 1.4 | 15 ± 0.86 | 19 ± 1.1 | 22 ± 2.1 | |
| Polyphenols | Tyr | >100 | >100 | >100 | >100 | >100 | >100 |
| HTyr | >100 | 82 ± 18 | >100 | 50 ± 25 | >100 | >100 | |
| Control | VP-16 | 1.5 ± 0.25 | 1.2 ± 0.30 | 2.4 ± 0.94 | 15 ± 1.5 | 18 ± 4.4 | 24 ± 2.6 |
| CDDP | 4.9 ± 0.2 | 1.9 ± 0.2 | 1.8 ± 0.5 | 2.7 ± 0.4 | 17 ± 3 | 26 ± 4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Begines, P.; Martos, S.; Lagunes, I.; Maya, I.; Padrón, J.M.; López, Ó.; Fernández-Bolaños, J.G. Chemoselective Preparation of New Families of Phenolic-Organoselenium Hybrids—A Biological Assessment. Molecules 2022, 27, 1315. https://doi.org/10.3390/molecules27041315
Begines P, Martos S, Lagunes I, Maya I, Padrón JM, López Ó, Fernández-Bolaños JG. Chemoselective Preparation of New Families of Phenolic-Organoselenium Hybrids—A Biological Assessment. Molecules. 2022; 27(4):1315. https://doi.org/10.3390/molecules27041315
Chicago/Turabian StyleBegines, Paloma, Sergio Martos, Irene Lagunes, Inés Maya, José M. Padrón, Óscar López, and José G. Fernández-Bolaños. 2022. "Chemoselective Preparation of New Families of Phenolic-Organoselenium Hybrids—A Biological Assessment" Molecules 27, no. 4: 1315. https://doi.org/10.3390/molecules27041315
APA StyleBegines, P., Martos, S., Lagunes, I., Maya, I., Padrón, J. M., López, Ó., & Fernández-Bolaños, J. G. (2022). Chemoselective Preparation of New Families of Phenolic-Organoselenium Hybrids—A Biological Assessment. Molecules, 27(4), 1315. https://doi.org/10.3390/molecules27041315