Phytochemicals of Avocado Residues as Potential Acetylcholinesterase Inhibitors, Antioxidants, and Neuroprotective Agents
Abstract
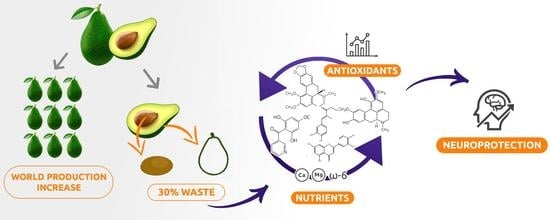
:1. Introduction
2. Results and Discussion
2.1. Residues Weight and Extracts Yields
2.2. Mineral Contents in Avocado Residues
2.3. Fatty Acid Composition
2.4. Determination of the Total Phenolic and Flavonoid Contents
2.5. Total Antioxidant Capacity and Antioxidant Activity Determined by DPPH Radical Scavenging and Ferric Reducing Power
2.6. Acetylcholinesterase Inhibition
2.7. Activity of P. americana Extracts in Neuroprotection Using D. melanogaster Model
2.8. Identification of Metabolites by Paper Spray Mass Spectrometry
3. Materials and Methods
3.1. Materials and Reagents
3.2. Biomass Processing and Extract Preparation
3.3. Mineral Element Analysis by Atomic Absorption
3.4. Fatty Acid Gas Chromatography Analysis
3.5. Total Content of Phenol and Flavonoids Assay
3.6. Antioxidant Activity
3.6.1. Total Antioxidant Capacity
3.6.2. Ferric Reducing Power Assay
3.6.3. Free Radical Capture Assay (DPPH)
3.7. Acetylcholinesterase Inhibition Assay
3.8. In Vivo Neuroprotection Activity Using Drosophila melanogaster Model
3.9. Paper Spray Mass Spectrometry
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ochoa-Zarzosa, A.; Báez-Magaña, M.; Guzmán-Rodríguez, J.J.; Flores-Alvarez, L.J.; Lara-Márquez, M.; Zavala-Guerrero, B.; Salgado-Garciglia, R.; López-Gómez, R.; López-Meza, J.E. Bioactive Molecules From Native Mexican Avocado Fruit (Persea americana var. drymifolia): A Review. Plant Foods Hum. Nutr. 2021, 76, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, T.; Ayele, M.; Gibril, M.; Ferede, E.; Limeneh, D.Y.; Kong, F. Beneficiation of avocado processing industry by-product: A review on future prospect. Curr. Res. Green Sustain. Chem. 2022, 5, 100253. [Google Scholar] [CrossRef]
- Alkhalaf, M.I.; Alansari, W.S.; Ibrahim, E.A.; ELhalwagy, M.E.A. Anti-oxidant, anti-inflammatory and anti-cancer activities of avocado (Persea americana) fruit and seed extract. J. King Saud Univ. Sci. 2019, 31, 1358–1362. [Google Scholar] [CrossRef]
- Borges, C.V.; Maraschin, M.; Coelho, D.S.; Leonel, M.; Gomez, H.A.G.; Belin, M.A.F.; Diamante, M.S.; Amorim, E.P.; Gianeti, T.; Castro, G.R.; et al. Nutritional value and antioxidant compounds during the ripening and after domestic cooking of bananas and plantains. Food Res. Int. 2020, 132, 109061. [Google Scholar] [CrossRef] [PubMed]
- Araujo, R.G.; Rodríguez-Jasso, R.M.; Ruíz, H.A.; Govea-Salas, M.; Pintado, M.; Aguilar, C.N. Recovery of bioactive components from avocado peels using microwave-assisted extraction. Food Bioprod. Process. 2021, 127, 152–161. [Google Scholar] [CrossRef]
- Baysal, S.S.; Ülkü, M.A. Food Loss and Waste: A Sustainable Supply Chain Perspective. ICGI-Global: Istanbul, Turkey, 2021; ISBN 9789251317549. [Google Scholar]
- Yap, K.M.; Sekar, M.; Seow, L.J.; Gan, S.H.; Bonam, S.R.; Mat Rani, N.N.I.; Lum, P.T.; Subramaniyan, V.; Wu, Y.S.; Fuloria, N.K.; et al. Mangifera indica (Mango): A promising medicinal plant for breast cancer therapy and understanding its potential mechanisms of action. Breast Cancer Targets Ther. 2021, 13, 471–503. [Google Scholar] [CrossRef]
- Béné, C.; Oosterveer, P.; Lamotte, L.; Brouwer, I.D.; de Haan, S.; Prager, S.D.; Talsma, E.F.; Khoury, C.K. When food systems meet sustainability—Current narratives and implications for actions. World Dev. 2019, 113, 116–130. [Google Scholar] [CrossRef]
- González-Chang, M.; Wratten, S.D.; Shields, M.W.; Costanza, R.; Dainese, M.; Gurr, G.M.; Johnson, J.; Karp, D.S.; Ketelaar, J.W.; Nboyine, J.; et al. Understanding the pathways from biodiversity to agro-ecological outcomes: A new, interactive approach. Agric. Ecosyst. Environ. 2020, 301, 107053. [Google Scholar] [CrossRef]
- Tumwesigye, K.S.; O’Brien, E.; Oliveira, J.C.; Crean, A.; Sousa-Gallagher, M.J. Engineered food supplement excipients from bitter cassava for minimisation of cassava processing waste in environment. Future Foods 2020, 1–2, 100003. [Google Scholar] [CrossRef]
- Ortega-Arellano, H.F.; Jimenez-Del-Rio, M.; Velez-Pardo, C. Neuroprotective Effects of Methanolic Extract of Avocado Persea americana (var. Colinred) Peel on Paraquat-Induced Locomotor Impairment, Lipid Peroxidation and Shortage of Life Span in Transgenic knockdown Parkin Drosophila melanogaster. Neurochem. Res. 2019, 44, 1986–1998. [Google Scholar] [CrossRef]
- Salazar-López, N.J.; Domínguez-Avila, J.A.; Yahia, E.M.; Belmonte-Herrera, B.H.; Wall-Medrano, A.; Montalvo-González, E.; González-Aguilar, G.A. Avocado fruit and by-products as potential sources of bioactive compounds. Food Res. Int. 2020, 138, 109774. [Google Scholar] [CrossRef] [PubMed]
- Del Castillo-Llamosas, A.; del Río, P.G.; Pérez-Pérez, A.; Yáñez, R.; Garrote, G.; Gullón, B. Recent advances to recover value-added compounds from avocado by-products following a biorefinery approach. Curr. Opin. Green Sustain. Chem. 2021, 28, 100433. [Google Scholar] [CrossRef]
- Fuloria, S.; Yusri, M.A.A.; Sekar, M.; Gan, S.H.; Rani, N.N.I.M.; Lum, P.T.; Ravi, S.; Subramaniyan, V.; Azad, A.K.; Jeyabalan, S.; et al. Genistein: A Potential Natural Lead Molecule for New Drug Design and Development for Treating Memory Impairment. Molecules 2022, 27, 265. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Action Plan on the Public Health Response to Dementia 2017–2025; World Health Organization: Geneva, Switzerland, 2017; p. 27. [Google Scholar]
- Bernstein, J.J.J.; Holt, G.B.; Bernstein, J. Price dispersion of generic medications. PLoS ONE 2019, 14, e0225280. [Google Scholar] [CrossRef] [PubMed]
- FAO; IFAD; UNICEF; WHO. The State of Food Security and Nutrition in the World 2020; FAO: Rome, Italy, 2020; ISBN 9789251329016. [Google Scholar] [CrossRef]
- Ferguson, E.L.; Watson, L.; Berger, J.; Chea, M.; Chittchang, U.; Fahmida, U.; Khov, K.; Kounnavong, S.; Le, B.M.; Rojroongwasinkul, N.; et al. Realistic Food-Based Approaches Alone May Not Ensure Dietary Adequacy for Women and Young Children in South-East Asia. Matern. Child Health J. 2019, 23, 55–66. [Google Scholar] [CrossRef]
- Adams, J.B.; Sorenson, J.C.; Pollard, E.L.; Kirby, J.K.; Audhya, T. Evidence-based recommendations for an optimal prenatal supplement for women in the U.S., part two: Minerals. Nutrients 2021, 13, 1849. [Google Scholar] [CrossRef] [PubMed]
- Lourenção, L.F.d.P.; de Paula, N.C.; Cardoso, M.A.; Santos, P.R.; de Oliveira, I.R.C.; Fonseca, F.L.A.; da Veiga, G.L.; Alves, B.d.C.A.; Graciano, M.M.d.C.; Pereira-Dourado, S.M. Biochemical markers and anthropometric profile of children enrolled in public daycare centers. J. Pediatr. 2021; in press. [Google Scholar] [CrossRef]
- Guirlanda, C.P.; da Silva, G.G.; Takahashi, J.A. Cocoa honey: Agro-industrial waste or underutilized cocoa by-product? Future Foods 2021, 4, 100061. [Google Scholar] [CrossRef]
- Mousa, M.M.H.; El-Magd, M.A.; Ghamry, H.I.; Alshahrani, M.Y.; El-Wakeil, N.H.M.; Hammad, E.M.; Asker, G.A.H. Pea peels as a value-added food ingredient for snack crackers and dry soup. Sci. Rep. 2021, 11, 22747. [Google Scholar] [CrossRef]
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, A.L.; Cena, H.; Ghiselli, A.; La Vecchia, C.; Lercker, G.; Manzato, E.; Pirillo, A.; et al. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Atherosclerosis 2020, 292, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Park, W.J. The Biochemistry and Regulation of Fatty Acid Desaturases in Animals; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128112304. [Google Scholar]
- Orsavova, J.; Misurcova, L.; Vavra Ambrozova, J.; Vicha, R.; Mlcek, J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int. J. Mol. Sci. 2015, 16, 2871. [Google Scholar] [CrossRef]
- Guillén-Sánchez, J.; Paucar-Menacho, L.M. Oxidative stability and shelf life of avocado oil extracted cold and hot using discard avocado (Persea americana). Sci. Agropecu. 2020, 11, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Velderrain-Rodríguez, G.R.; Quero, J.; Osada, J.; Martín-Belloso, O.; Rodríguez-Yoldi, M.J. Phenolic-rich extracts from avocado fruit residues as functional food ingredients with antioxidant and antiproliferative properties. Biomolecules 2021, 11, 997. [Google Scholar] [CrossRef] [PubMed]
- Vinha, A.F.; Moreira, J.; Barreira, S.V.P. Physicochemical Parameters, Phytochemical Composition and Antioxidant Activity of the Algarvian Avocado (Persea americana Mill.). J. Agric. Sci. 2013, 5, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Amado, D.A.V.; Helmann, G.A.B.; Detoni, A.M.; de Carvalho, S.L.C.; de Aguiar, C.M.; Martin, C.A.; Tiuman, T.S.; Cottica, S.M. Antioxidant and antibacterial activity and preliminary toxicity analysis of four varieties of avocado (Persea americana Mill.). Braz. J. Food Technol. 2019, 22, 04418. [Google Scholar] [CrossRef]
- Tremocoldi, M.A.; Rosalen, P.L.; Franchin, M.; Massarioli, A.P.; Denny, C.; Daiuto, É.R.; Paschoal, J.A.R.; Melo, P.S.; De Alencar, S.M. Exploration of avocado by-products as natural sources of bioactive compounds. PLoS ONE 2018, 13, e0192577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez, E.V.; Tovar, J.; Mosquera, O.M.; Cardozo, F. Actividad Neuroprotectora de Solanum ovalifolium (SOlanaceae) contra la toxicidad Inducida por rotenona em Drosophila melanogaster. Rev Fac Cienc. Basicas 2017, 13, 27–35. [Google Scholar] [CrossRef]
- Siima, A.A.; Stephano, F.; Munissi, J.J.E.; Nyandoro, S.S. Ameliorative effects of flavonoids and polyketides on the rotenone induced Drosophila model of Parkinson’s disease. NeuroToxicology 2020, 81, 209–215. [Google Scholar] [CrossRef]
- Kumar, S.; Behl, T.; Sehgal, A.; Chigurupati, S.; Singh, S.; Mani, V.; Aldubayan, M.; Alhowail, A.; Kaur, S.; Bhatia, S.; et al. Exploring the focal role of LRRK2 kinase in Parkinson’s disease. Environ. Sci. Pollut. Res. 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.; Klein, C. UnCover Cellular Pathways Underlying Parkinson’s Disease. Cells 2021, 10, 579. [Google Scholar] [CrossRef]
- Bolus, H.; Crocker, K.; Boekhoff-Falk, G.; Chtarbanova, S. Modeling neurodegenerative disorders in drosophila melanogaster. Int. J. Mol. Sci. 2020, 21, 3055. [Google Scholar] [CrossRef]
- McBride, E.M.; Mach, P.M.; Dhummakupt, E.S.; Dowling, S.; Carmany, D.O.; Demond, P.S.; Rizzo, G.; Manicke, N.E.; Glaros, T. Paper spray ionization: Applications and perspectives. TrAC Trends Anal. Chem. 2019, 118, 722–730. [Google Scholar] [CrossRef]
- Silva, V.D.M.; Macedo, M.C.C.; dos Santos, A.N.; Silva, M.R.; Augusti, R.; Lacerda, I.C.A.; Melo, J.O.F.; Fante, C.A. Bioactive activities and chemical profile characterization using paper spray mass spectrometry of extracts of Eriobotrya japonica Lindl. leaves. Rapid Commun. Mass Spectrom. 2020, 34, e8883. [Google Scholar] [CrossRef] [PubMed]
- García, Y.M.; Ramos, A.L.C.C.; de Oliveira Júnior, A.H.; de Paula, A.C.C.F.F.; de Melo, A.C.; Andrino, M.A.; Silva, M.R.; Augusti, R.; de Araújo, R.L.B.; de Lemos, E.E.P.; et al. Physicochemical Characterization and Paper Spray Mass Spectrometry Analysis of Myrciaria Floribunda (H. West ex Willd.) O. Berg Accessions. Molecules 2021, 26, 7206. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, C.D.; de Paula, A.C.C.F.F.; de Oliveira Júnior, A.H.; Mendonça, H.d.O.P.; Reina, L.D.C.B.; Augusti, R.; Figueiredo-Ribeiro, R.d.C.L.; Melo, J.O.F. Paper spray mass spectrometry on the analysis of phenolic compounds in rhynchelytrum repens: A tropical grass with hypoglycemic activity. Plants 2021, 10, 1617. [Google Scholar] [CrossRef] [PubMed]
- Weremfo, A.; Adulley, F.; Adarkwah-Yiadom, M. Simultaneous Optimization of Microwave-Assisted Extraction of Phenolic Compounds and Antioxidant Activity of Avocado (Persea americana Mill.) Seeds Using Response Surface Methodology. J. Anal. Methods Chem. 2020, 2020, 7541927. [Google Scholar] [CrossRef] [PubMed]
- Segovia, F.J.; Hidalgo, G.I.; Villasante, J.; Ramis, X.; Almajano, M.P. Avocado seed: A comparative study of antioxidant content and capacity in protecting oil models from oxidation. Molecules 2018, 23, 2421. [Google Scholar] [CrossRef] [Green Version]
- Mijangos-Ramos, I.F.; Zapata-Estrella, H.E.; Ruiz-Vargas, J.A.; Escalante-Erosa, F.; Gómez-Ojeda, N.; García-Sosa, K.; Cechinel-Filho, V.; Meira-Quintão, N.L.; Peña-Rodríguez, L.M. Bioactive dicaffeoylquinic acid derivatives from the root extract of Calea urticifolia. Rev. Bras. Farmacogn. 2018, 28, 339–343. [Google Scholar] [CrossRef]
- Rosero, J.C.; Cruz, S.; Osorio, C.; Hurtado, N. Analysis of Phenolic Composition of Byproducts (Seeds and Peels) of Avocado (Persea americana Mill.) Cultivated in Colombia. Molecules 2019, 24, 3209. [Google Scholar] [CrossRef] [Green Version]
- Castro-López, C.; Bautista-Hernández, I.; González-Hernández, M.D.; Martínez-Ávila, G.C.G.; Rojas, R.; Gutiérrez-Díez, A.; Medina-Herrera, N.; Aguirre-Arzola, V.E. Polyphenolic Profile and Antioxidant Activity of Leaf Purified Hydroalcoholic Extracts from Seven Mexican Persea americana Cultivars. Molecules 2019, 24, 173. [Google Scholar] [CrossRef] [Green Version]
- Moita, I.S.; Yamaguchi, K.K.L.; Alcântara, J.M.; Silva, Y.C.; Fernandes, N.S.; Nakamura, C.V.; Veiga Junior, V.F. Phytochemical and Biological Studies on Ocotea ceanothifolia (Nees) Mez. Rev. Virtual Quim. 2019, 11, 1267–1276. [Google Scholar] [CrossRef]
- Alagbaoso, C.A.; Osakwe, O.S.; Tokunbo, I.I. Changes in proximate and phytochemical compositions of Persea americana mill. (avocado pear) seeds associated with ripening. J. Med. Biomed. Res. 2017, 16, 28–34. [Google Scholar]
- Setyawan, H.Y.; Sukardi, S.; Puriwangi, C.A. Phytochemicals properties of avocado seed: A review. IOP Conf. Ser. Earth Environ. Sci. 2021, 733, 012090. [Google Scholar] [CrossRef]
- Silva, Y.C.D. Estudo de Marcadores em Espécies de Aniba (Lauraceae) Bioativas da Amazônia. 2018. Available online: https://tede.ufam.edu.br/handle/tede/6494 (accessed on 23 January 2022).
- Nunes, G.B.L.; Costa, L.M.; Gutierrez, S.J.C.; Satyal, P.; De Freitas, R.M. Behavioral tests and oxidative stress evaluation in mitochondria isolated from the brain and liver of mice treated with riparin A. Life Sci. 2015, 121, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues de Carvalho, A.M.; Vasconcelos, L.F.; Moura Rocha, N.F.; Vasconcelos Rios, E.R.; Dias, M.L.; Maria de França Fonteles, M.; Gaspar, D.M.; Barbosa Filho, J.M.; Chavez Gutierrez, S.J.; Florenço de Sousa, F.C. Antinociceptive activity of Riparin II from Aniba riparia: Further elucidation of the possible mechanisms. Chem.-Biol. Interact. 2018, 287, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Chaves, R.d.C.; Mallmann, A.S.V.; de Oliveira, N.F.; Capibaribe, V.C.C.; da Silva, D.M.A.; Lopes, I.S.; Valentim, J.T.; Barbosa, G.R.; de Carvalho, A.M.R.; de Fonteles, M.M.F.; et al. The neuroprotective effect of Riparin IV on oxidative stress and neuroinflammation related to chronic stress-induced cognitive impairment. Horm. Behav. 2020, 122, 104758. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Pathak, N.; Fatima, E.; Negi, A.S. Plant isoquinoline alkaloids: Advances in the chemistry and biology of berberine. Eur. J. Med. Chem. 2021, 226, 113839. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Famta, P.; Famta, M.; Mehta, M.; Satija, S. Potential anti-epileptic phytoconstituents: An updated review. J. Ethnopharmacol. 2021, 268, 113565. [Google Scholar] [CrossRef] [PubMed]
- Martins, B.d.A.; Sande, D.; Solares, M.D.; Takahashi, J.A. Antioxidant role of morusin and mulberrofuran B in ethanol extract of Morus alba roots. Nat. Prod. Res. 2021, 35, 5993–5996. [Google Scholar] [CrossRef]
- Sande, D.; Colen, G.; dos Santos, G.F.; Ferraz, V.P.; Takahashi, J.A. Production of omega 3, 6, and 9 fatty acids from hydrolysis of vegetable oils and animal fat with Colletotrichum gloeosporioides lipase. Food Sci. Biotechnol. 2018, 27, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.O.; Labanca, R.A. Development and Chemical Characterization of Pequi Pericarp Flour. J. Braz. Chem. Soc. 2022, 00, 1–11, (in press). [Google Scholar]
- Bashmil, Y.M.; Ali, A.; Bk, A.; Dunshea, F.R.; Suleria, H.A.R. Screening and characterization of phenolic compounds from Australian grown bananas and their antioxidant capacity. Antioxidants 2021, 10, 1521. [Google Scholar] [CrossRef] [PubMed]
- Seyrekoglu, F.; Temiz, H.; Eser, F.; Yildirim, C. Comparison of the antioxidant activities and major constituents of three Hypericum species (H. perforatum, H. scabrum and H. origanifolium) from Turkey. S. Afr. J. Bot. 2022, 146, 723–727. [Google Scholar] [CrossRef]





| Minerals (mg/100 g of Sample) | Peels 1 | Seeds 1 |
|---|---|---|
| Ca | 26.78 ± 2.06 | 41.14 ± 8.50 |
| Cu | 0.20 ± 0.74 | 0.48 ± 0.01 |
| Fe | 0.72 ± 2.06 | 1.04 ± 7.40 |
| Mg | 23.87 ± 3.09 | 31.41 ± 1.82 |
| Mn | 4.23 ± 10.34 | 1.80 ± 6.48 |
| Zn | 0.67 ± 7.02 | 1.11 ± 0.57 |
| Fatty Acids Contents (%) | ||||
|---|---|---|---|---|
| Fatty Acids | Peels | Seeds | ||
| PEL-H | PEL-ET | SED-H | SED-ET | |
| Miristic 14:0 | 0.7 | 1.7 | 0.7 | 1.5 |
| Palmitic 16:0 | 42.5 | 47.9 | 23.0 | 22.2 |
| Palmitoleic 16:1 | 2.7 | 1.8 | 2.9 | 3.2 |
| Stearic 18:0 | 7.0 | 22.2 | 4.1 | 14.7 |
| Oleic 18:1 | 18.2 | 2.5 | 17.3 | 16.2 |
| Linoleic 18:2 | 4.5 | 0.7 | 34.8 | 27.4 |
| Linolenic 18:3 | 1.0 | 0.4 | 3.0 | 1.6 |
| Total saturated fatty acids | 50.2 | 71.8 | 27.8 | 38.4 |
| Total unsaturated fatty acids | 26.4 | 5.4 | 58 | 48.4 |
| Total | 76.6 | 77.2 | 85.8 | 86.8 |
| Assays | Extracts | |||
|---|---|---|---|---|
| PEL-H | PEL-ET | SED-H | SED-ET | |
| Total phenolic content (TPC) * | 26.33 ± 0.48 g | 35.40 ± 0.60 d | 32.48 ± 2.00 e | 32.15 ± 0.39 fe |
| Total flavonoid content (TFC) ** | 1243.78 ± 32.33 j | 694.058 ±1.490 l | 1199.04 ± 49.39 k | 640.72 ± 9.30 l |
| Assays | Extracts | |||
|---|---|---|---|---|
| PEL-H | PEL-ET | SED-H | SED-ET | |
| Total antioxidant capacity * | 26.33 ± 0.48 g | 35.40 ± 0.60 d | 32.48 ± 2.00 e | 32.15 ± 0.39 fe |
| DPPH scavenging (%) | 7.77 ± 1.44 o | 52.20 ± 1.05 m | 35.89 ± 1.59 n | 37.60 ± 1.67 n |
| Ferric reducing power (%) ** | 4.81 ± 1.37 g | 1.11 ± 0.25 i | 4.07 ± 1.21 gh | 2.38 ± 0.24 hi |
| AChE inhibition (%) | 70.8 ± 9.7 rq | 85.6 ± 11.1 pq | 65.0 ± 8.9 s | 78.0 ± 6.8 qr |
| m/z | Compound | Chemical Structure | Class | MS/MS | Extract | Reference |
|---|---|---|---|---|---|---|
| 151 | Vanillin | C8H8O3 | Aldehyde | 136, 107, 93 | S, P | [43] |
| 179 | Caffeic acid | C9H8O4 | Phenolic acid | 151, 135 | S | [43] |
| 191 | Quinic acid | C7H12O6 | Phenolic acid | 93, 111 | S, P | [43] |
| 197 | Syringic acid | C9H10O5 | Phenolic acid | 153, 141, 125 | S | [43] |
| 209 | 5-Hydroxyferulic acid | C10H10O5 | Phenolic acid | 191, 165, 118 | S | [44] |
| 223 | Sinapic acid | C11H12O5 | Phenolic acid | 179, 151, 85 | S | [43] |
| 269 | Apigenin | C15H10O5 | Flavonoid | 252, 223, 197 | S, P | [43] |
| 285 | Kaempferol | C15H10O6 | Flavonoid | 255, 224, 213 | S, P | [43] |
| 289 | Catechin | C15H13O6 | Flavonoid | 274, 245, 217, 199 | S, P | [44] |
| 301 | Quercetin | C15H10O7 | Flavonoid | 272, 265, 123 | S, P | [43] |
| 315 | Hydroxytyrosol hexoside | C14H20O8 | Phenolic glycoside | 297, 269, 243 | S, P | [44] |
| 325 | p-coumaroyl hexose | C15H17O8 | Phenolic glycoside | 261, 197, 183, 170 | S, P | [44] |
| 337 | 3-O-p-coumaroylquinic acid | C16H18O8 | Phenolic compound | 293, 237, 183 | S, P | [44] |
| 341 | Caffeic acid-hexoside | C15H18O9 | Phenolic glycoside | 280, 185, 183, 179 | S, P | [44] |
| 353 | 5-O-caffeoylquinic acid | C16H18O9 | Phenolic compound | 309, 211, 191, 183 | S, P | [43] |
| 417 | Kaempferol-O-pentoside | C20H18O10 | Flavonoid glycoside | 404, 344, 289 | S, P | [44] |
| 419 | Cyanidin 3-O-pentatoside | C20H19O10 | Flavonoid glycoside | 395, 361, 292, 287 | S, P | [44] |
| 431 | Vitexin | C21H20O10 | Flavonoid glycoside | 362, 351, 311, 196 | S | [44] |
| 433 | Peonidin 3-O-pentoside | C21H21O11 | Flavonoid glycoside | 420, 389, 301, 205 | S, P | [44] |
| 435 | Phloridzin | C21H24O10 | Flavonoid glycoside | 426, 416, 369 | S | [43] |
| 447 | Kaempferol-O-hexoside | C21H19O11 | Flavonoid glycoside | 420, 403, 352, 301 | S, P | [44] |
| 449 | Dihydroquercetin-3,5-rhamnoside | C21H22O11 | Flavonoid glycoside | 430, 303, 298, 286 | S | [44] |
| 451 | Cinchonain | C24H20O9 | Flavonoid | 424, 414, 377 | S, P | [44] |
| 461 | Isorhamnetin-O-coumaroyl | C22H22O11 | Flavonoid | 461, 417, 216 | S, P | [44] |
| 463 | Quercetin-3-hexoside | C21H20O12 | Flavonoid glycoside | 464, 384, 316, 300 | S | [44] |
| 473 | Quercetin-3-O-hexoside | C21H19O12 | Flavonoid glycoside | 467, 436, 372 | S, P | [45] |
| 477 | Quercetin glucuronide | C21H18O13 | Flavonoid | 431, 262, 231 | S | [44] |
| 487 | Caffeoyl hexose-deoxyhexoside | C22H31O12 | Flavonoid | 442, 298, 173 | S | [44] |
| 491 | Dimethyl ellagic acid hexoside | C22H22O13 | Flavonoid | 343, 275, 269 | S, P | [44] |
| 563 | Apigenin-C-hexoside-C-pentoside | C26H28O14 | Flavonoid glycoside | 531, 446, 298 | S | [44] |
| 575 | Procyanidin dimer A | C30H24O12 | Flavonoid | 431, 404, 329 | S | [43] |
| 577 | Procyanidin dimer B | C30H25O12 | Flavonoid | 532, 516, 420 | S | [44] |
| 579 | Luteolin 7-O-(2”-O-pentosyl)hexoside | C26H28O15 | Flavonoid glycoside | 560, 542, 514 | S, P | [44] |
| 593 | Catechin dihexoside | C27H29O15 | Flavonoid glycoside | 574, 495, 347 | S | [44] |
| 595 | Quercetin-O-pentatosyl-hexoside | C26H28O16 | Flavonoid glycoside | 562, 558, 497 | S, P | [44] |
| 609 | Rutin | C27H30O16 | Flavonoid glycoside | 573, 564, 208 | S, P | [44] |
| 625 | Quercetin-3,4’-O-diglucoside | C27H30O17 | Flavonoid glycoside | 605, 588, 581 | S, P | [43] |
| 863 | Procyanidin trimer A | C45H36O18 | Flavonoid | 845, 826, 555 | S, P | [43] |
| 865 | Procyanidin trimer B-isomer 1 | C45H38O18 | Flavonoid | 829, 735, 560 | S | [43] |
| m/z | Compound | Chemical Structure | MS/MS | Extract | Reference |
|---|---|---|---|---|---|
| 204 | Anibine (1) | C11H9NO3 | 183, 188, 192 | S | [48] |
| 244 | Duckeine (2) | C13H11NO4 | 226, 235, 187 | S | [48] |
| 256 | Riparin I (3) | C16H17NO2 | 241, 187, 212 | S, P | [48] |
| 270 | Norcanelilline (6) | C17H19NO3 | 214, 261, 240 | S, P | [48] |
| 272 | Riparin II (4) | C16H17NO3 | 263, 250, 254 | S, P | [48] |
| 282 | Anicanine (8) | C19H23NO3 | 200, 183, 192 | S | [48] |
| 288 | Riparin III (5) | C16H17NO4 | 270, 106, 271 | S, P | [48] |
| 296 | (-)-α-8-methyl-pseudoanibacanine (9) | C19H21NO3 | 279, 287, 239 | S, P | [48] |
| 300 | N-methylcoclaurine (7) | C18H21NO3 | 283, 291, 227 | S, P | [48] |
| 305 | Ceceline (10) | C19H16N2O2 | 263, 273, 287 | S, P | [48] |
| 312 | (+)-Manibacanine (11) | C19H21NO3 | 116, 291, 243 | S, P | [48] |
| 325 | Cassythicine (12) | C19H19NO4 | 287, 316, 307 | S, P | [45] |
| 328 | Isoboldine (13) | C19H21NO4 | 297, 178, 310 | S, P | [48] |
| 330 | Reticuline (14) | C19H23NO4 | 187, 218, 235 | S, P | [48] |
| 339 | Nantenine (15) | C20H21NO4 | 249, 330, 321 | S, P | [45] |
| 424 | Anibamine (16) | C30H50N+ | 334, 379, 418 | S, P | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, G.G.; Pimenta, L.P.S.; Melo, J.O.F.; Mendonça, H.d.O.P.; Augusti, R.; Takahashi, J.A. Phytochemicals of Avocado Residues as Potential Acetylcholinesterase Inhibitors, Antioxidants, and Neuroprotective Agents. Molecules 2022, 27, 1892. https://doi.org/10.3390/molecules27061892
da Silva GG, Pimenta LPS, Melo JOF, Mendonça HdOP, Augusti R, Takahashi JA. Phytochemicals of Avocado Residues as Potential Acetylcholinesterase Inhibitors, Antioxidants, and Neuroprotective Agents. Molecules. 2022; 27(6):1892. https://doi.org/10.3390/molecules27061892
Chicago/Turabian Styleda Silva, Geisa Gabriela, Lúcia Pinheiro Santos Pimenta, Júlio Onésio Ferreira Melo, Henrique de Oliveira Prata Mendonça, Rodinei Augusti, and Jacqueline Aparecida Takahashi. 2022. "Phytochemicals of Avocado Residues as Potential Acetylcholinesterase Inhibitors, Antioxidants, and Neuroprotective Agents" Molecules 27, no. 6: 1892. https://doi.org/10.3390/molecules27061892
APA Styleda Silva, G. G., Pimenta, L. P. S., Melo, J. O. F., Mendonça, H. d. O. P., Augusti, R., & Takahashi, J. A. (2022). Phytochemicals of Avocado Residues as Potential Acetylcholinesterase Inhibitors, Antioxidants, and Neuroprotective Agents. Molecules, 27(6), 1892. https://doi.org/10.3390/molecules27061892