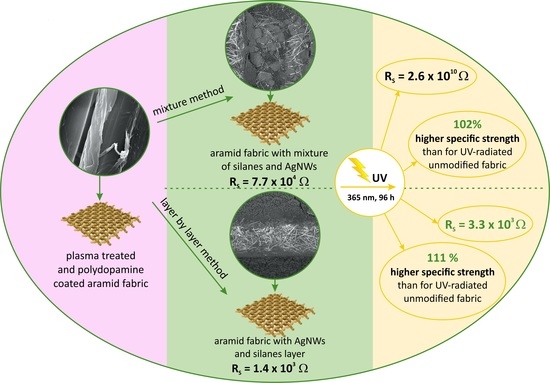
Silver Nanowires and Silanes in Hybrid Functionalization of Aramid Fabrics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Functionalization Methods
Preparation of Silver Nanowires (AgNWs) Colloid
Low-Pressure Air RF Plasma Treatment of Aramid Fabrics
Polydopamine Functionalization of Aramid Fabrics
Silanes Sol Preparation
AgNWs and Silanes Functionalization of Aramid Fabrics
UV Irradiation
2.2.2. Characterization Methods
SEM/EDS Analysis
Optical Microscopy
FTIR Analysis
Surface Properties
Specific Strength
Abrasion Resistance
Conductivity
3. Results and Discussion
3.1. Modification of the Aramid Fabrics with Low-Pressure Air RF Plasma and Polydopamine
3.2. AgNWs and Silanes Modified Aramid Fabrics before and after UV Irradiation
3.3. Surface Properties
3.4. The Durability before and after UV Irradiation
3.4.1. Specific Strength
3.4.2. Abrasion Resistance
3.5. Conductive Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhang, H.; Liang, G.; Gu, A.; Yuan, L. Facile preparation of hyperbranched polysiloxane-grafted aramid fibers with simulta-neously improved UV resistance, surface activity, and thermal and mechanical properties. Ind. Eng. Chem. Res. 2014, 53, 2684–2696. [Google Scholar] [CrossRef]
- Wang, H.; Xie, H.; Hu, Z.; Wu, D.; Chen, P. The influence of UV radiation and moisture on the mechanical properties and micro-structure of single Kevlar fibre using optical methods. Polym. Degrad. Stab. 2012, 97, 1755–1761. [Google Scholar] [CrossRef]
- Zhu, J.; Yuan, L.; Guan, Q.; Liang, G.; Gu, A. A novel strategy of fabricating high performance UV-resistant aramid fibers with simultaneously improved surface activity, thermal and mechanical properties through building polydopamine and graphene oxide bi-layer coatings. Chem. Eng. J. 2017, 310, 134–147. [Google Scholar] [CrossRef]
- Foksowicz-Flaczyk, J.; Walentowska, J.; Przybylak, M.; Maciejewski, H. Multifunctional durable properties of textile materials modified by biocidal agents in the sol-gel process. Surf. Coat. Technol. 2016, 304, 160–166. [Google Scholar] [CrossRef]
- Stoppa, M.; Chiolerio, A. Wearable Electronics and Smart Textiles: A Critical Review. Sensors 2014, 14, 11957–11992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gowri, S.; Almeida, L.; Amorim, T.; Carneiro, N.; Souto, A.P.; Esteves, M.F. Polymer nanocomposites for multifunctional fin-ishing of textiles—A review. Text. Res. J. 2014, 80, 1290–1306. [Google Scholar] [CrossRef]
- Nejman, A.; Kamińska, I.; Jasińska, I.; Celichowski, G.; Cieślak, M. Influence of Low-Pressure RF Plasma Treatment on Aramid Yarns Properties. Molecules 2020, 25, 3476. [Google Scholar] [CrossRef]
- Inagaki, N.; Tasaka, S.; Kawai, H. Surface modification of Kevlar® fiber by a combination of plasma treatment and coupling agent treatment for silicone rubber composite. J. Adhes. Sci. Technol. 1992, 6, 279–291. [Google Scholar] [CrossRef]
- Sun, Z.; Zhou, Y.; Li, W.; Chen, S.; You, S.; Ma, J. Preparation of Silver-Plated Para-Aramid Fiber by Employing Low-Temperature Oxygen Plasma Treatment and Dopamine Functionalization. Coatings 2019, 9, 599. [Google Scholar] [CrossRef] [Green Version]
- Sa, R.; Yan, Y.; Wei, Z.; Zhang, L.; Wang, W.; Tian, M. Surface Modification of Aramid Fibers by Bio-Inspired Poly(dopamine) and Epoxy Functionalized Silane Grafting. ACS Appl. Mater. Interfaces 2014, 6, 21730–21738. [Google Scholar] [CrossRef]
- Chai, D.; Xie, Z.; Wang, Y.; Liu, L.; Yum, Y.-J. Molecular Dynamics Investigation of the Adhesion Mechanism Acting between Dopamine and the Surface of Dopamine-Processed Aramid Fibers. ACS Appl. Mater. Interfaces 2014, 6, 17974–17984. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Liu, X.; Chen, X.; Soutis, C. Surface Modification of Aramid Fibres with Graphene Oxide for Interface Improvement in Composites. Appl. Compos. Mater. 2018, 25, 843–852. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Zhang, F.; Li, J.; Li, B.; Zhao, C. Oxidant-induced dopamine polymerization for multifunctional coatings. Polym. Chem. 2010, 1, 1430–1433. [Google Scholar] [CrossRef]
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-molecule mechanics of mussel adhesion. Proc. Natl. Acad. Sci. USA 2006, 103, 12999–13003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burzio, L.A.; Waite, J.H. Cross-Linking in Adhesive Quinoproteins: Studies with Model Decapeptides. Biochemistry 2000, 39, 11147–11153. [Google Scholar] [CrossRef] [PubMed]
- Sever, M.J.; Weisser, J.T.; Monahan, J.; Srinivasan, S.; Wilker, J.J. Metal-mediated cross-linking in the generation of a ma-rine-mussel adhesive. Angew. Chem. Int. Ed. 2004, 43, 448–450. [Google Scholar] [CrossRef]
- Yu, M.; Hwang, J.; Deming, T.J. Role of l-3,4-Dihydroxyphenylalanine in Mussel Adhesive Proteins. J. Am. Chem. Soc. 1999, 121, 5825–5826. [Google Scholar] [CrossRef]
- Gu, T.; Zhu, D.; Lu, S. Surface Functionalization of Silver-Coated Aramid Fiber. Polym. Sci. Ser. A 2020, 62, 196–204. [Google Scholar] [CrossRef]
- Wang, W.; Li, R.; Tian, M.; Liu, L.; Zou, H.; Zhao, X.; Zhang, L. Surface Silverized Meta-Aramid Fibers Prepared by Bio-inspired Poly(dopamine) Functionalization. ACS Appl. Mater. Interfaces 2013, 5, 2062–2069. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, L.; Xue, J.; Hao, L.; Li, J.; He, Y.; Cheng, B. A Novel Two-Step Method for Fabricating Silver Plating Cotton Fabrics. J. Nanomater. 2016, 2016, 2375836. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Gates, B.; Mayers, B.; Xia, Y. Crystalline Silver Nanowires by Soft Solution Processing. Nano. Lett. 2002, 2, 165–168. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Chen, X.; Wan, J.; Qian, Y. A Simple Hydrothermal Route to Large-Scale Synthesis of Uniform Silver Nanowires. Chem.—Eur. J. 2004, 11, 160–163. [Google Scholar] [CrossRef] [PubMed]
- James, S.; Robinson, A.; Arnold, J.; Worsley, D. The effects of humidity on photodegradation of poly(vinyl chloride) and polyethylene as measured by the CO2 evolution rate. Polym. Degrad. Stab. 2013, 98, 508–513. [Google Scholar] [CrossRef] [Green Version]
- Mather, R.R.; Wardman, R.H. The Chemistry of Textile Fibers; RSC Publishing: Cambridge, UK, 2011. [Google Scholar]
- Lin, M.C.; Chu, C.J.; Tsai, L.C.; Lin, H.Y.; Wu, C.S.; Wu, Y.P.; Shieh, D.B.; Su, Y.W.; Chen, C.D. Control and Detection of Organosilane Polarization on Nanowire Field-Effect Transistors. Nano Lett. 2007, 7, 3656–3661. [Google Scholar] [CrossRef] [Green Version]
- Chiavari, C.; Balbo, A.; Zanotto, F.; Vassura, I.; Bignozzi, M.C.; Monticelli, C. Organosilane coatings applied on bronze: In-fluence of UV radiation and thermal cycles on the protectiveness. Progr. Org. Coat. 2015, 82, 91–100. [Google Scholar] [CrossRef]
- Shi, F.; Xu, J.; Zhang, Z. Study on UV-protection and hydrophobic properties of cotton fabric functionalized by graphene oxide and silane coupling agent. Pigment Resin Technol. 2019, 48, 237–242. [Google Scholar] [CrossRef]
- Tragoonwichian, S.; O’Rear, E.A.; Yanumet, N. Grafting polymerization of 2-[3-(2h-benzotriazol-2-yl)-4-hydroxyphenyl]ethyl methacrylate on vinyltriethoxysilane-treated cotton for the preparation of ultraviolet-protective fabrics. J. Appl. Polym. Sci. 2009, 114, 62–69. [Google Scholar] [CrossRef]
- Roe, B.; Zhang, X. Durable Hydrophobic Textile Fabric Finishing Using Silica Nanoparticles and Mixed Silanes. Text. Res. J. 2009, 79, 1115–1122. [Google Scholar] [CrossRef]
- Zeng, X.; Xu, G.; Gao, Y.; An, Y. Surface Wettability of (3-Aminopropyl)triethoxysilane Self-Assembled Monolayers. J. Phys. Chem. B 2010, 115, 450–454. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Q.; Li, X.; Xie, H.; Nie, C. Facile synthesis of ternary flexible silica aerogels with coarsened skeleton for oil–water separation. RSC Adv. 2020, 10, 42297–42304. [Google Scholar] [CrossRef]
- Souza, K.G.D.S.; Cotting, F.; Aoki, I.V.; Amado, F.D.R.; Capelossi, V.R. Study of the wettability and the corrosion protection of the hybrid silane (3-aminopropyl) triethoxysilane (APTES) and (3-glycidyloxypropyl) trimethoxysilane (GPTMS) film on galvannealed steel. Matéria 2020, 25. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Far, H.S.; Haji, A.; Rosace, G. Facile fabrication of breathable and superhydrophobic fabric based on silica na-noparticles and amino-modified polydimethylsiloxane. Preprints 2020, 257, 5491–5498. [Google Scholar]
- Liu, H.; Lee, Y.-Y.; Norsten, T.B.; Chong, K. In situ formation of anti-bacterial silver nanoparticles on cotton textiles. J. Ind. Text. 2013, 44, 198–210. [Google Scholar] [CrossRef]
- Giesz, P.; Mackiewicz, E.; Nejman, A.; Celichowski, G.; Cieślak, M. Investigation on functionalization of cotton and viscose fabrics with AgNWs. Cellulose 2016, 24, 409–422. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, H.; Witkowska, B.; Kamińska, I.; Twarowska-Schmidt, K.; Wierus, K.; Puchowicz, D. Comparison of the rates of polypropylene fibre degradation caused by artificial light and sunlight. Fib. Tex. East. Eur. 2011, 4, 53–58. [Google Scholar]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Cieslak, M.; Puchowicz, D.; Schmidt, H. Evaluation of the possibility of using surface free energy study to design protective fabrics. Text. Res. J. 2011, 82, 1177–1189. [Google Scholar] [CrossRef]
- Giesz, P.; Celichowski, G.; Puchowicz, D.; Kamińska, I.; Grobelny, J.; Batory, D.; Cieślak, M. Microwave-assisted TiO2: Anatase formation on cotton and viscose fabric surfaces. Cellulose 2016, 23, 2143–2159. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, H.; Cieślak, M. Concrete with carpet recyclates: Suitability assessment by surface energy evaluation. Waste Manag. 2008, 28, 1182–1187. [Google Scholar] [CrossRef]
- Sabdin, S.; Azmi, M.A.M.; Badruzaman, N.A.; Makmon, F.Z.; Aziz, A.A.; Said, N.A.M. Effect of APTES Percentage towards Reduced Graphene Oxide Screen Printed Electrode Surface for Biosensor Application. Mater. Today Proc. 2019, 19, 1183–1188. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Zhu, L.; Zhang, H.; Zhu, B.; Xu, Y. Antifouling and Antimicrobial Polymer Membranes Based on Bioinspired Polydopamine and Strong Hydrogen-Bonded Poly(N-vinyl pyrrolidone). ACS Appl. Mater. Interfaces 2013, 5, 12895–12904. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, L.; Sun, M.; Zhang, X.; Chen, W. Surface modification of polypropylene nonwoven fabrics by grafting of polydopamine. Adv. Polym. Technol. 2018, 37, 3519–3528. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Bai, S.; Chen, T.; Yang, H.; Guo, X. Facile preparation of flexible and highly stable graphene oxide-silver nanowire hybrid transparent conductive electrode. Mater. Res. Express 2019, 7, 016413. [Google Scholar] [CrossRef] [Green Version]












| Fabric | Mass per Unit Area, g/m2 | Mass per Unit Area of AgNW Coating, g/m2 | Mass per Unit Area of Silanes Coating, g/m2 | Mass per Unit Area of AgNWs and Silanes Coating, g/m2 |
|---|---|---|---|---|
| mAr | 205 ± 2 | |||
| mAr/RF/PD/1Ag | 214 ± 6 | 9 ± 3 | ||
| mAr/RF/PD/5Ag | 248 ± 7 | 43 ± 2 | ||
| mAr/RF/PD/5Ag+S | 273 ± 7 | 68 ± 5 | ||
| mAr/RF/PD/5Ag/S | 305 ± 9 | 43 ± 2 | 62 ± 1 | 105 ± 3 |
| pAr | 165 ± 3 | |||
| pAr/RF/PD/1Ag | 171 ± 4 | 6 ± 1 | ||
| pAr/RF/PD/5Ag | 200 ± 1 | 35 ± 3 | ||
| pAr/RF/PD/5Ag+S | 222 ± 5 | 57 ± 2 | ||
| pAr/RF/PD/5Ag/S | 263 ± 4 | 35 ± 3 | 63 ± 5 | 98 ± 6 |
| Liquid | Surface Tension, mJ/m2 | ||
|---|---|---|---|
| γl | γld | γlp | |
| Water (distilled) | 72.8 | 21.8 | 51.0 |
| Formamide (99.5%, Sigma-Aldrich, UK) | 58.0 | 39.0 | 19.0 |
| Diiodomethane (99%, Sigma-Aldrich, UK) | 50.8 | 48.5 | 2.3 |
| pAr Fabric | Reference Polydopamine Film | ||
|---|---|---|---|
| Bands, cm−1 | Description [7,10,34] | Bands, cm−1 | Description [7,10,34] |
| 698 | C–H out of plane substituted aromatic ring | 698 | C–H out of plane substituted aromatic ring |
| 821 | p-substituted phenyl | 911 | CONH bending, C–N stretching |
| 1306 | C–N stretching | 1034 | C–O stretching |
| 1509 | C=C stretching | 1113 | C–H bending |
| 1538 | N–H bending | 1335 | C–N stretching |
| 1637 | C=O stretching | 1413 | C=O stretching |
| 1740 | C=O stretching | 1440 | C=C stretching vibrations of an aromatic ring |
| 3323 | N–H stretching | 1527 | C=N stretching vibrations of an aromatic ring |
| 1594 | C=C stretching vibrations of an aromatic ring, C–N stretching | ||
| 3150 | N–H stretching | ||
| Fabric | Weight Percentage of Elements, wt.% | ||||
|---|---|---|---|---|---|
| C | N | O | Si | Ag | |
| mAr 1 | 68 ± 1 | 10 ± 1 | 21 ± 1 | ||
| mAr/RF/PD/1Ag 2 | 68 ± 1 | 8 ± 1 | 20 ± 1 | 3 ± 1 | |
| mAr/RF/PD/5Ag 2 | 56 ± 2 | 7 ± 1 | 18 ± 1 | 18 ± 2 | |
| mAr/RF/PD/5Ag+S 2 | 54 ± 1 | 6 ± 1 | 28 ± 1 | 5 ± 1 | 6 ± 1 |
| mAr/RF/PD/5Ag/S 2 | 41 ± 1 | 8 ± 1 | 31 ± 1 | 7 ± 1 | 12 ± 1 |
| pAr 1 | 66 ± 1 | 13 ± 1 | 19 ± 1 | ||
| pAr/RF/PD/1Ag 1,2 | 66 ± 1 | 6 ± 1 | 20 ± 1 | 6 ± 1 | |
| pAr/RF/PD/5Ag 1,2 | 54 ± 3 | 5 ± 1 | 18 ± 1 | 21 ± 3 | |
| pAr/RF/PD/5Ag+S 1,2 | 54 ± 1 | 5 ± 1 | 26 ± 1 | 5 ± 1 | 8 ± 1 |
| pAr/RF/PD/5Ag/S 1,2 | 42 ± 1 | 7 ± 1 | 31 ± 1 | 6 ± 1 | 12 ± 1 |
| Fabric | Contact Angle [o] | ||
|---|---|---|---|
| ΘW | ΘF | ΘDIM | |
| mAr | 64 ± 2 | 58 ± 2 | 63 ± 2 |
| mAr/RF | 19 ± 3 | 36 ± 3 | 27 ± 3 |
| mAr/RF/PD | 0 ± 0 | 36 ± 1 | 90 ± 0 |
| mAr/RF/PD/1Ag | 77 ± 2 | 74 ± 3 | 36 ± 4 |
| mAr/RF/PD/5Ag | 87 ± 1 | 90 ± 2 | 32 ± 3 |
| mAr/RF/PD/5Ag+S | 125 ± 5 | 106 ± 2 | 55 ± 2 |
| mAr/RF/PD/5Ag/S | 112 ± 2 | 96 ± 2 | 51 ± 1 |
| pAr | 77 ± 4 | 33 ± 2 | 14 ± 3 |
| pAr/RF | 12 ± 5 | 18 ± 5 | 5 ± 1 |
| pAr/RF/PD | 0 ± 0 | 19 ± 1 | 68 ± 1 |
| pAr/RF/PD/1Ag | 84 ± 4 | 77 ± 2 | 36 ± 3 |
| pAr/RF/PD/5Ag | 89 ± 3 | 85 ± 2 | 34 ± 4 |
| pAr/RF/PD/5Ag+S | 120 ± 1 | 106 ± 3 | 58 ± 2 |
| pAr/RF/PD/5Ag/S | 114 ± 2 | 100 ± 1 | 53 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nejman, A.; Baranowska-Korczyc, A.; Ranoszek-Soliwoda, K.; Jasińska, I.; Celichowski, G.; Cieślak, M. Silver Nanowires and Silanes in Hybrid Functionalization of Aramid Fabrics. Molecules 2022, 27, 1952. https://doi.org/10.3390/molecules27061952
Nejman A, Baranowska-Korczyc A, Ranoszek-Soliwoda K, Jasińska I, Celichowski G, Cieślak M. Silver Nanowires and Silanes in Hybrid Functionalization of Aramid Fabrics. Molecules. 2022; 27(6):1952. https://doi.org/10.3390/molecules27061952
Chicago/Turabian StyleNejman, Alicja, Anna Baranowska-Korczyc, Katarzyna Ranoszek-Soliwoda, Izabela Jasińska, Grzegorz Celichowski, and Małgorzata Cieślak. 2022. "Silver Nanowires and Silanes in Hybrid Functionalization of Aramid Fabrics" Molecules 27, no. 6: 1952. https://doi.org/10.3390/molecules27061952
APA StyleNejman, A., Baranowska-Korczyc, A., Ranoszek-Soliwoda, K., Jasińska, I., Celichowski, G., & Cieślak, M. (2022). Silver Nanowires and Silanes in Hybrid Functionalization of Aramid Fabrics. Molecules, 27(6), 1952. https://doi.org/10.3390/molecules27061952