Photocatalytic CO2 Reduction Using TiO2-Based Photocatalysts and TiO2 Z-Scheme Heterojunction Composites: A Review
Abstract
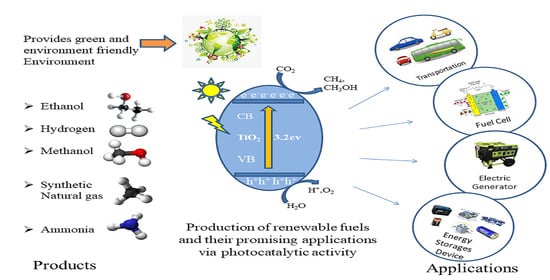
:1. Introduction
- It is not expensive, while production on a large scale is cost effective.
- It has excellent ability to resist corrosion as well as having good photo stability.
- Many physical and chemical techniques have been well developed already to synthesize the porous film and nanoparticulate powder of TiO2.
- It has exhibited excellent photocatalytic efficiency.
- It can be activated in visible sunlight and can start a chemical reaction.
2. The Fundamentals of Photocatalytic CO2 Reduction
2.1. Basic Principle of Photocatalysis
2.2. Thermodynamics of Photocatalytic Reduction of CO2
2.2.1. Effect of Temperature
2.2.2. Effect of Light
2.2.3. Effect of CB and VB Potential
2.3. Recombination of Charges and Effect of Metal-Modified Surface
3. Synthesis Methods of TiO2-Based Photocatalyst
3.1. Sol-Gel Synthesis
3.2. Co-Precipitation Method
3.3. Impregnation Method
3.4. Hydrothermal Synthesis
3.5. One-Pot Synthesis
3.6. Other Methods
4. TiO2-Based Photocatalysts for CO2 Reduction
4.1. Doping
4.1.1. Metal-Doped TiO2 Photocatalysts
4.1.2. Non-Metal-Doped TiO2 Photocatalysts
4.1.3. Defect Chemistry of Doped TiO2
4.2. Modification Using Metal Oxides (MO)
4.3. Formation of Nanomaterials
4.4. Crystal Phases
4.5. Modification by Surface Photosensitization
| Photocatalyst Name | Reactants | T, P | Light Source | Major Product: (Formation Rate µmol h−1 gcar−1) | Reactor Type | Ref. | |
|---|---|---|---|---|---|---|---|
| TiO2 Powder | CO2 | H2O | P = 6.5 MPa | 990 W, Xe Lamp | HCOOH | High-pressure Stainless-steel Vessel, Pyrex cell H-shaped | [105] |
| Cu/TiO2 | CO2 | H2O | T = 273–323 K, P = 1 atm | 75 W, High-Pressure Mercury Lamp, λ > 280 nm | CH4, CH3OH, CO | Quartz cell | [106] |
| Cu/TiO2 | CO2 | H2O | 25 °C, 1 atm | 450 W, Xe-lamp | Ethylene, Methane | Stainless steel with quartz window | [107] |
| TiO2 | CO2 | H2O | NA | Hg Lamp, UV light, Ultrahigh pressure, 500 W, λ = 350 nm | CH4 | Slurry Reactor | [108] |
| TiO2 | CO2 | H2O | 273 K | 8 W, λ = 254 nm, Hg lamp | CH4 | Quartz tube Reactor | [109] |
| Thin film, Pt (1 nm)-TiO2 | Mixture CO2 | H2O vapor | NA | 400 W, Xe lamp, (250–388 nm) | CH4: 1361 | Flow Reactor System | [110] |
| CuO-TiO2 (hollow microspheres) | CO2 | H2O | P = 3.45 bar | Hg Lamp, 40 W, 254 nm | CO: 14.54, CH4: 2.07 | Closed System | [111] |
| TiO2 (P25) | Saturated CO2 | H2O | T = 278 K | HP Hg arc, 500 W | CO: 0.35 | Photo-Kolbe reaction of acetic acid | [112] |
| Au/TiO2 | CO2 | H2O | P = 2 bar | 6 W lamp | CH4: 8.0 | Steel Reactor | [113] |
| Fe/TiO2 | CO2 | H2 + H2O | 343 K | Xe Lamp | CO: 8.2 | Cylindrical Vessel Reactor | [114] |
| Pd/TiO2 | CO2 | H2 | NA | 150 W, Hg lamp | CH4: 355.6, CO: 46.3, C2H6: 39.6 | Miniature Visual Autoclave | [115] |
| Pd/TiO2 | CO2 | H2O | NA | 500 W Hg lamp | CH4:1.415, CO: 0.722 | Stainless steel chamber | [116] |
| Ag/TiO2 | CO2 | H2O | 25 °C, 1 atm | 8 W Hg Lamp | CH3OH: 9.0, CH4: 8.5 | Stirred batch annular slurry reactor | [117] |
| TiO2/zeolite | CO2 | H2O | P = 10−6 torr | 75 W high-pressure lamp | CH4 | Quartz cell with a flat bottom | [106] |
| MoS2/TiO2 nanosheets | -- | H2O | P = Atmosphere | 300 W Xe lamp | CH3OH: 10.6 | Airtight quartz glass reactor | [118] |
| MoS2/TiO2 fibers | -- | H2O | NA | 350 W Xe lamp | CH4: 2.86 | Homemade apparatus | [97] |
| 2D/2D SnS2/TiO2 | -- | H2O | P = 1 MPa | 300 W Xe lamp | CH4:23 | 50 mL stainless steel reactor with quartz flakes | [119] |
| CdS/TiO2 film | --- | H2O | NA | 300 W Xe lamp | CH4: 11.9 | Under simulated sunlight irradiation (from a 300 W Xenon arc lamp) | [120] |
| CdS(Bi2S3)/TiO2 | -- | H2O | NA | 500 W Xe lamp with cutoff Filter | CH3OH | Continuous-flow reactor | [121] |
| ZnIn2S4/TiO2 nanobelts | -- | H2O | NA | 300 W Xe lamp | CH4: 1.135 | Gas tight system | [122] |
| CuGaS2/RGO/TiO2 | -- | H2O | P = 1 atm | 300 W Xe lamp | CO: 0.15 | Batch-type top irradiation cell with a Pyrex window | [123] |
5. TiO2 Z-Scheme Heterojunction Composites for CO2 Photoreduction
6. Challenges and Recommendations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef]
- Tollefson, J. How green is my future? UN panel foresees big growth in renewable energy, but policies will dictate just how big. Nature 2011, 473, 134–136. [Google Scholar] [CrossRef]
- Wang, Y.; He, D.; Chen, H.; Wang, D. Catalysts in electro-, photo-and photoelectrocatalytic CO2 reduction reactions. J. Photochem. Photobiol. C Photochem. Rev. 2019, 40, 117–149. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Guo, L.; Zhang, X.; Ribeiro, C.; He, T. Solar-heating boosted catalytic reduction of CO2 under full-solar spectrum. Chin. J. Catal. 2019, 41, 131–139. [Google Scholar] [CrossRef]
- Xiao, J.; Yang, W.; Gao, S.; Sun, C.; Li, Q. Fabrication of ultrafine ZnFe2O4 nanoparticles for efficient photocatalytic reduction CO2 under visible light illumination. J. Mater. Sci. Technol. 2018, 34, 2331–2336. [Google Scholar] [CrossRef]
- Ahmed, N.; Shibata, Y.; Taniguchi, T.; Izumi, Y. Photocatalytic conversion of carbon dioxide into methanol using zinc–copper–M (III)(M= aluminum, gallium) layered double hydroxides. J. Catal. 2011, 279, 123–135. [Google Scholar] [CrossRef]
- Morikawa, M.; Ogura, Y.; Ahmed, N.; Kawamura, S.; Mikami, G.; Okamoto, S.; Izumi, Y. Photocatalytic conversion of carbon dioxide into methanol in reverse fuel cells with tungsten oxide and layered double hydroxide photocatalysts for solar fuel generation. Catal. Sci. Technol. 2014, 4, 1644–1651. [Google Scholar] [CrossRef]
- Kubacka, A.; Fernández-García, M.; Colón, G. Advanced nanoarchitectures for solar photocatalytic applications. Chem. Rev. 2011, 112, 1555–1614. [Google Scholar] [CrossRef]
- Meyer, G. The 2010 Millennium Technology Grand Prize: Dye-Sensitized Solar Cells. ACS Nano 2010, 4, 4337–4343. [Google Scholar] [CrossRef]
- Morris, A.J.; Meyer, G.J.; Fujita, E. Molecular Approaches to the Photocatalytic Reduction of Carbon Dioxide for Solar Fuels. Acc. Chem. Res. 2009, 42, 1983–1994. [Google Scholar] [CrossRef]
- Tahir, M.; Amin, S.N. Recent advances in the photocatalytic conversion of carbon dioxide to fuels with water and/or hydrogen using solar energy and beyond. Energy Con. Mang. 2013, 257, 171–186. [Google Scholar]
- Singh, A.; Chang, S.L.Y.; Hocking, R.K.; Bach, U.; Spiccia, L. Highly active nickel oxide water oxidation catalysts deposited from molecular complexes. Energy Environ. Sci. 2012, 6, 579–586. [Google Scholar] [CrossRef]
- El-Khouly, M.E.; El-Mohsnawy, E.; Fukuzumi, S. Solar energy conversion: From natural to artificial photosynthesis. J. Photochem. Photobiol. C Photochem. Rev. 2017, 31, 36–83. [Google Scholar] [CrossRef]
- Zhan, W.; Sun, L.; Han, X. Recent progress on engineering highly efficient porous semiconductor photocatalysts derived from metal–organic frameworks. Nano-Micro Lett. 2019, 11, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zhao, H.; Andino, J.M.; Li, Y. Photocatalytic CO2 reduction with H2O on TiO2 nanocrystals: Comparison of anatase, rutile, and brookite polymorphs and exploration of surface chemistry. ACS Catal. 2012, 2, 1817–1828. [Google Scholar] [CrossRef]
- Yu, J.; Low, J.; Xiao, W.; Zhou, P.; Jaroniec, M. Enhanced photocatalytic CO2-reduction activity of anatase TiO2 by coexposed {001} and {101} facets. J. Am. Chem. Soc. 2014, 136, 8839–8842. [Google Scholar] [CrossRef]
- Ganesh, I. Electrochemical conversion of carbon dioxide into renewable fuel chemicals—The role of nanomaterials and the commercialization. Renew. Sustain. Energy Rev. 2016, 59, 1269–1297. [Google Scholar] [CrossRef]
- Ganesh, I. Conversion of carbon dioxide into methanol—A potential liquid fuel: Fundamental challenges and opportunities (A review). Renew. Sustain. Energy Rev. 2014, 31, 221–257. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Hawecker, J.; Lehn, J.-M.; Ziessel, R. Efficient photochemical reduction of CO2 to CO by visible light irradiation of systems containing Re(bipy)(CO)3X or Ru(bipy)32+–Co2+combinations as homogeneous catalysts. J. Chem. Soc. Chem. Commun. 1983, 9, 536–538. [Google Scholar] [CrossRef]
- Hawecker, J.; Lehn, J.M.; Ziessel, R. Photochemical and electrochemical reduction of carbon dioxide to carbon monoxide mediated by (2, 2′-bipyridine) tricarbonylchlororhenium (I) and related complexes as homogeneous catalysts. Helv. Chim. Acta 1986, 69, 1990–2012. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Takeda, H.; Ishitani, O. Photocatalytic reduction of CO2 using metal complexes. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 106–137. [Google Scholar] [CrossRef]
- Ramesha, G.K.; Brennecke, J.F.; Kamat, V. Origin of catalytic effect in the reduction of CO2 at nanostructured TiO2 films. ACS Catal. 2014, 4, 3249–3254. [Google Scholar] [CrossRef]
- Yuan, L.; Xu, Y.-J. Photocatalytic conversion of CO2 into value-added and renewable fuels. Appl. Surf. Sci. 2015, 342, 154–167. [Google Scholar] [CrossRef]
- Yu, J.; Wang, K.; Xiao, W.; Cheng, B. Photocatalytic reduction of CO2 into hydrocarbon solar fuels over g-C3N4–Pt nanocomposite photocatalysts. Phys. Chem. Chem. Phys. 2014, 16, 11492–11501. [Google Scholar] [CrossRef]
- Ehsan, M.F.; He, T. In situ synthesis of ZnO/ZnTe common cation heterostructure and its visible-light photocatalytic reduction of CO2 into CH4. Appl. Catal. B Environ. 2015, 166–167, 345–352. [Google Scholar] [CrossRef]
- Marszewski, M.; Cao, S.; Yu, J.; Jaroniec, M. Semiconductor-based photocatalytic CO2 conversion. Mater. Horizons 2014, 2, 261–278. [Google Scholar] [CrossRef]
- Hong, J.; Zhang, W.; Ren, J.; Xu, R. Photocatalytic reduction of CO2: A brief review on product analysis and systematic methods. Anal. Methods 2013, 5, 1086–1097. [Google Scholar] [CrossRef]
- Doustkhah, E.; Assadi, M.H.N.; Komaguchi, K.; Tsunoji, N.; Esmat, M.; Fukata, N.; Tomita, O.; Abe, R.; Ohtani, B.; Ide, Y. In situ Blue titania via band shape engineering for exceptional solar H2 production in rutile TiO2. Appl. Catal. B Environ. 2021, 297, 120380. [Google Scholar] [CrossRef]
- Mani, D.; Tahawy, R.; Doustkhah, E.; Shanmugam, M.; Arivanandhan, M.; Jayavel, R.; Ide, Y. A rutile TiO2 nanobundle as a precursor of an efficient visible-light photocatalyst embedded with Fe2O3. Inorg. Chem. Front. 2021, 8, 4423–4430. [Google Scholar] [CrossRef]
- Chen, P.; Lei, B.; Dong, X.; Wang, H.; Sheng, J.; Cui, W.; Li, J.; Sun, Y.; Wang, Z.; Dong, F. Rare-earth single-atom La–N charge-transfer bridge on carbon nitride for highly efficient and selective photocatalytic CO2 reduction. ACS Nano 2020, 14, 15841–15852. [Google Scholar] [CrossRef] [PubMed]
- Kaempf, G. Degradation Processes in TiO2-Pigmented Paint Films on Exposure to Weathering. J. Paint Tech. 1974, 46, 56–63. [Google Scholar]
- Van Driel, B.A.; Berg, K.J.V.D.; Smout, M.; Dekker, N.; Kooyman, P.; Dik, J. Investigating the effect of artists’ paint formulation on degradation rates of TiO2-based oil paints. Heritage Sci. 2018, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Artesani, A.; Mosca, S.; Dozzi, M.V.; Valentini, G.; Comelli, D. Determination of crystal phases in mixed TiO2 paint films by non-invasive optical spectroscopies. Microchem. J. 2020, 155, 104739. [Google Scholar] [CrossRef]
- Rompelberg, C.; Heringa, M.B.; Van Donkersgoed, G.; Drijvers, J.; Roos, A.; Westenbrink, S.; Peters, R.; Van Bemmel, G.; Brand, W.; Oomen, A.G. Oral intake of added titanium dioxide and its nanofraction from food products, food supplements and toothpaste by the Dutch population. Nanotoxicology 2016, 10, 1404–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popov, A.; Priezzhev, A.V.; Lademann, J.; Myllylä, R. TiO2nanoparticles as an effective UV-B radiation skin-protective compound in sunscreens. J. Phys. D Appl. Phys. 2005, 38, 2564–2570. [Google Scholar] [CrossRef]
- Jaroenworaluck, A.; Sunsaneeyametha, W.; Kosachan, N.; Stevens, R. Characteristics of silica-coated TiO2 and its UV absorption for sunscreen cosmetic applications. Surf. Interface Anal. 2006, 38, 473–477. [Google Scholar] [CrossRef]
- Velimirovic, M.; Wagner, S.; Monikh, F.A.; Uusimäki, T.; Kaegi, R.; Hofmann, T.; von der Kammer, F. Accurate quantification of TiO2 nanoparticles in commercial sunscreens using standard materials and orthogonal particle sizing methods for verification. Talanta 2020, 215, 120921. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Ji, L.; Li, L.; Ji, X.; Zhang, Y.; Mou, S.; Wu, T.; Liu, Q.; Li, B.; Zhu, X.; Luo, Y.; et al. Highly selective electrochemical reduction of CO2 to alcohols on an FeP nanoarray. Angew. Chem. 2020, 132, 768–772. [Google Scholar] [CrossRef]
- Vasileff, A.; Zhu, Y.; Zhi, X.; Zhao, Y.; Ge, L.; Chen, H.M.; Zheng, Y.; Qiao, S. Electrochemical reduction of CO2 to ethane through stabilization of an ethoxy intermediate. Angew. Chem. 2020, 132, 19817–19821. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, Y.-T.; Vispute, T.; Xiao, R.; Huber, G.W. Catalytic Conversion of Biomass-derived Feedstocks into Olefins and Aromatics with ZSM-5: The Hydrogen to Carbon Effective Ratio. Energy Environ. Sci. 2011, 4, 2297–2307. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Daud, W.M.A.W. A review on advances in photocatalysts towards CO2 conversion. RSC Adv. 2014, 4, 20856–20893. [Google Scholar] [CrossRef]
- Das, K.; De, S.K. Optical Properties of the Type-II Core−Shell TiO2@CdS Nanorods for Photovoltaic Applications. J. Phys. Chem. C 2009, 113, 3494–3501. [Google Scholar] [CrossRef]
- Robert, D. These Uses of CO2 Could Cut Emissions—And Make Trillions of Dollars. 2019. Available online: https://www.vox.com/energy-and-environment/2019/11/13/20839531/climate-change-industry-co2-carbon-capture-utilization-storage-ccu (accessed on 14 November 2020).
- Sohn, Y.; Huang, W.; Taghipour, F. Recent progress and perspectives in the photocatalytic CO2 reduction of Ti-oxide-based nanomaterials. Appl. Surf. Sci. 2017, 396, 1696–1711. [Google Scholar] [CrossRef]
- Low, J.; Cheng, B.; Yu, J. Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2: A review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- Guo, L.-J.; Wang, Y.-J.; He, T. Photocatalytic reduction of CO2 over heterostructure semiconductors into value-added chemicals. Chem. Rec. 2016, 16, 1918–1933. [Google Scholar] [CrossRef]
- Li, K.; An, X.; Park, K.H.; Khraisheh, M.; Tang, J. A critical review of CO2 photoconversion: Catalysts and reactors. Catal. Today 2014, 224, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Shehzad, N.; Tahir, M.; Johari, K.; Murugesan, T.; Hussain, M. A critical review on TiO2 based photocatalytic CO2 reduction system: Strategies to improve efficiency. J. CO2 Util. 2018, 26, 98–122. [Google Scholar] [CrossRef]
- Graham, J.; Hammer, N. Photocatalytic Water Splitting and Carbon Dioxide Reduction, Handbook of Climate Change Mitigation; Springer: Oxford, MA, USA, 2012. [Google Scholar]
- Li, K.; Peng, B.; Peng, T. Recent advances in heterogeneous photocatalytic CO2 conversion to solar fuels. ACS Catal. 2016, 6, 7485–7527. [Google Scholar] [CrossRef]
- Karamian, E.; Sharifnia, S. On the general mechanism of photocatalytic reduction of CO2. J. CO2 Util. 2016, 16, 194–203. [Google Scholar] [CrossRef]
- Nikokavoura, A.; Trapalis, C. Alternative photocatalysts to TiO2 for the photocatalytic reduction of CO2. Appl. Surf. Sci. 2017, 391, 149–174. [Google Scholar] [CrossRef]
- Kang, Y.-F.; Li, Y.-H.; Fang, Y.-W.; Xu, Y.; Wei, X.-M.; Yin, X.-B. Carbon quantum dots for zebrafish fluorescence imaging. Sci. Rep. 2015, 5, srep11835. [Google Scholar] [CrossRef]
- Wang, J.; Lin, S.; Tian, N.; Ma, T.; Zhang, Y.; Huang, H. Nanostructured metal sulfides: Classification, modification strategy, and solar-driven CO2 reduction application. Adv. Funct. Mater. 2021, 31, 2008008. [Google Scholar] [CrossRef]
- Jiao, X.; Zheng, K.; Liang, L.; Li, X.; Sun, Y.; Xie, Y. Fundamentals and challenges of ultrathin 2D photocatalysts in boosting CO2 photoreduction. Chem. Soc. Rev. 2020, 49, 6592–6604. [Google Scholar] [CrossRef]
- Koch, S.W.; Kira, M.; Khitrova, G.; Gibbs, H.M. Semiconductor excitons in new light. Nat. Mater. 2006, 5, 523–531. [Google Scholar] [CrossRef]
- Zürch, M.; Chang, H.-T.; Borja, L.J.; Kraus, P.; Cushing, S.K.; Gandman, A.; Kaplan, C.; Oh, M.H.; Prell, J.S.; Prendergast, D.; et al. Direct and simultaneous observation of ultrafast electron and hole dynamics in germanium. Nat. Commun. 2017, 8, 15734. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhao, X.; Terashima, C.; Fujishima, A.; Nakata, K. Thermodynamic and kinetic analysis of heterogeneous photocatalysis for semiconductor systems. Phys. Chem. Chem. Phys. 2014, 16, 8751–8760. [Google Scholar] [CrossRef]
- Albero, J.; Garcia, H.; Corma, A. Temperature dependence of solar light assisted CO2 reduction on Ni based photocatalyst. Top. Catal. 2016, 59, 787–791. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.-L.; Ong, W.-J.; Chai, S.-P.; Mohamed, A.R. Photocatalytic reduction of CO2 with H2O over graphene oxide-supported oxygen-rich TiO2 hybrid photocatalyst under visible light irradiation: Process and kinetic studies. Chem. Eng. J. 2017, 308, 248–255. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Lin, S.D.; Wu, J.C.-S.; Bai, H. Artificial sunlight and ultraviolet light induced photo-epoxidation of propylene over V-Ti/MCM-41 photocatalyst. Beilstein J. Nanotechnol. 2014, 5, 566–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krejcikova, S.; Matějová, L.; Kočí, K.; Obalová, L.; Matěj, Z.; Čapek, L.; Šolcová, O. Preparation and characterization of Ag-doped crystalline titania for photocatalysis applications. Appl. Catal. B Environ. 2012, 111–112, 119–125. [Google Scholar] [CrossRef]
- Masakazu, A. Photocatalytic reduction of CO2 with H2O on highly dispersed Tioxide catalysts as a model of artificial photosynthesis. J. CO2 Util. 2013, 1, 8–17. [Google Scholar]
- Wu, J.C.S.; Lin, H.-M. Photo reduction of CO2 to methanol via TiO2 photocatalyst. Int. J. Photoenergy 2005, 7, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Tahir, M.; Amin, N.S. Advances in visible light responsive titanium oxide-based photocatalysts for CO2 conversion to hydrocarbon fuels. Energy Convers. Manag. 2013, 76, 194–214. [Google Scholar] [CrossRef]
- Tseng, I.-H.; Wu, J.C.S.; Chou, H.-Y. Effects of sol–gel procedures on the photocatalysis of Cu/TiO2 in CO2 photoreduction. J. Catal. 2004, 221, 432–440. [Google Scholar] [CrossRef]
- Tennakone, K. Photoreduction of carbonic acid by mercury coated n-titanium dioxide. Sol. Energy Mater. 1984, 10, 235–238. [Google Scholar] [CrossRef]
- Ishitani, O.; Inoue, C.; Suzuki, Y. Photocatalytic reduction of carbon dioxide to methane and acetic acid by an aqueous suspension of metal-deposited TiO2. J. Photochem. Photobiol. A Chem. 1993, 72, 269–271. [Google Scholar] [CrossRef]
- Matsuoka, M.; Anpo, M. Local structures, excited states, and photocatalytic reactivities of highly dispersed catalysts constructed within zeolites. J. Photochem. Photobiol. C Photochem. Rev. 2003, 3, 225–252. [Google Scholar] [CrossRef]
- Thampi, K.R.; Kiwi, J.; Grätzel, M. Methanation and photo-methanation of carbon dioxide at room temperature and atmospheric pressure. Nature 1987, 327, 506–508. [Google Scholar] [CrossRef]
- Usubharatana, P.; McMartin, D.; Veawab, A.; Tontiwachwuthikul, P. Photocatalytic process for CO2 emission reduction from industrial flue gas streams. Ind. Eng. Chem. Res. 2006, 45, 2558–2568. [Google Scholar] [CrossRef]
- Xia, X.-H.; Jia, Z.-J.; Yu, Y.; Liang, Y.; Wang, Z.; Ma, L.-L. Preparation of multi-walled carbon nanotube supported TiO2 and its photocatalytic activity in the reduction of CO2 with H2O. Carbon 2007, 45, 717–721. [Google Scholar] [CrossRef]
- Tseng, I.-H.; Wu, J.C.-S. Chemical states of metal-loaded titania in the photoreduction of CO2. Catal. Today 2004, 97, 113–119. [Google Scholar] [CrossRef]
- Nishimura, A.; Mitsui, G.; Hirota, M.; Hu, E. CO2 reforming performance and visible light responsibility of Cr-doped TiO2 prepared by sol-gel and dip-coating method. Int. J. Chem. Eng. 2010, 2010, 309103. [Google Scholar] [CrossRef] [Green Version]
- Ong, W.-J.; Gui, M.M.; Chai, S.-P.; Mohamed, A.R. Direct growth of carbon nanotubes on Ni/TiO2 as next generation catalysts for photoreduction of CO2 to methane by water under visible light irradiation. RSC Adv. 2013, 3, 4505–4509. [Google Scholar] [CrossRef]
- Gui, M.M.; Chai, S.-P.; Mohamed, A.R. Modification of MWCNT@ TiO2 core–shell nanocomposites with transition metal oxide dopants for photoreduction of carbon dioxide into methane. Appl. Surf. Sci. 2014, 319, 37–43. [Google Scholar] [CrossRef]
- Kohno, Y.; Hayashi, H.; Takenaka, S.; Tanaka, T.; Funabiki, T.; Yoshida, S. Photo-enhanced reduction of carbon dioxide with hydrogen over Rh/TiO2. J. Photochem. Photobiol. A Chem. 1999, 126, 117–123. [Google Scholar] [CrossRef]
- Gui, M.M.; Wong, W.M.P.; Chai, S.-P.; Mohamed, A.R. One-pot synthesis of Ag-MWCNT@ TiO2 core–shell nanocomposites for photocatalytic reduction of CO2 with water under visible light irradiation. Chem. Eng. J. 2015, 278, 272–278. [Google Scholar] [CrossRef]
- Gui, M.M.; Chai, S.-P.; Xu, B.-Q.; Mohamed, A.R. Enhanced visible light responsive MWCNT/TiO2 core–shell nanocomposites as the potential photocatalyst for reduction of CO2 into methane. Sol. Energy Mater. Sol. Cells 2014, 122, 183–189. [Google Scholar] [CrossRef]
- Umebayashi, T.; Yamaki, T.; Itoh, H.; Asai, K. Band gap narrowing of titanium dioxide by sulfur doping. Appl. Phys. Lett. 2002, 81, 454–456. [Google Scholar] [CrossRef]
- Dette, C.; Osorio, M.A.P.; Kley, C.S.; Punke, P.; Patrick, C.; Jacobson, P.; Giustino, F.; Jung, S.J.; Kern, K. TiO2 anatase with a bandgap in the visible region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef] [PubMed]
- Adekoya, D.; Tahir, M.; Amin, N.A.S. Recent trends in photocatalytic materials for reduction of carbon dioxide to methanol. Renew. Sustain. Energy Rev. 2019, 116, 109389. [Google Scholar] [CrossRef]
- Li, J.; Hou, X.; Sun, T.; Han, J.; Liu, H.; Li, D. Hydrophilic, antibacterial and photocatalytic properties of TiO2 composite films modified by the methods of N+ ion implantation and doping of CNTs under visible light irradiation. Surf. Coat. Technol. 2019, 365, 123–128. [Google Scholar] [CrossRef]
- Zikriya, M.; Nadaf, Y.; Bharathy, P.V.; Renuka, C. Luminescent characterization of rare earth Dy3+ ion doped TiO2 prepared by simple chemical co-precipitation method. J. Rare Earths 2018, 37, 24–31. [Google Scholar] [CrossRef]
- Liu, E.; Kang, L.; Wu, F.; Sun, T.; Hu, X.; Yang, Y.; Liu, H.; Fan, J. Photocatalytic reduction of CO2 into methanol over Ag/TiO2 nanocomposites enhanced by surface plasmon resonance. Plasmonics 2013, 9, 61–70. [Google Scholar] [CrossRef]
- Chen, R.; Pang, S.; An, H.; Dittrich, T.; Fan, F.; Li, C. Giant defect-induced effects on nanoscale charge separation in semiconductor photocatalysts. Nano Lett. 2018, 19, 426–432. [Google Scholar] [CrossRef]
- Maarisetty, D.; Baral, S.S. Defect-induced enhanced dissociative adsorption, optoelectronic properties and interfacial contact in Ce doped TiO2: Solar photocatalytic degradation of Rhodamine B. Ceram. Int. 2019, 45, 22253–22263. [Google Scholar] [CrossRef]
- Slamet; Nasution, W.H.; Purnama, E.; Kosela, S.; Gunlazuardi, J. Photocatalytic reduction of CO2 on copper-doped Titania catalysts prepared by improved-impregnation method. Catalysis Commun. 2005, 6, 313–319. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Nguyen, D.L.T.; Nguyen, V.-H.; Le, T.-H.; Vo, D.-V.N.; Trinh, Q.T.; Bae, S.-R.; Chae, S.Y.; Kim, S.Y.; Van Le, Q. Recent advances in TiO2-based photocatalysts for reduction of CO2 to fuels. Nanomaterials 2020, 10, 337. [Google Scholar] [CrossRef] [Green Version]
- Maznichenko, D. 3-D Fibrous Network of TiO2 Nanoparticles: Raman Sensor Development. Master’s Thesis, Ryerson University, Toronto, ON, Canada, 2012. [Google Scholar]
- Tsai, C.-W.; Chen, H.M.; Liu, R.-S.; Asakura, K.; Chan, T.-S. Ni@ NiO core–shell structure-modified nitrogen-doped InTaO4 for solar-driven highly efficient CO2 reduction to methanol. J. Phys. Chem. C 2011, 115, 10180–10186. [Google Scholar] [CrossRef]
- Huang, Y.; Yan, C.-F.; Guo, C.-Q.; Huang, S.-L. Enhanced photoreduction activity of carbon dioxide over Co3O4/CeO2 catalysts under visible light irradiation. Int. J. Photoenergy 2015, 2015, 230808. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Huang, B.; Liu, Y.; Wang, Z.; Qin, X.; Zhang, X.; Dai, Y. An anion exchange approach to Bi2WO6 hollow microspheres with efficient visible light photocatalytic reduction of CO2 to methanol. Chem. Commun. 2012, 48, 9729–9731. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Yu, J.; Xu, H.; Hu, X.; Luo, X.; Yang, L.; Tu, X. Synthesis of hierarchical flower-like Bi2MoO6microspheres as efficient photocatalyst for photoreduction of CO2into solar fuels under visible light. CrystEngComm 2016, 18, 3472–3480. [Google Scholar] [CrossRef]
- Xu, F.; Zhu, B.; Cheng, B.; Yu, J.; Xu, J. 1D/2D TiO2/MoS2 hybrid nanostructures for enhanced photocatalytic CO2 reduction. Adv. Opt. Mater. 2018, 6, 1800911. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, T.; Konishi, T.; Tada, H.; Tohge, N.; Nishii, J.; Ito, S. A patterned TiO2 (anatase)/TiO2 (rutile) bilayer-type photocatalyst: Effect of the anatase/rutile junction on the photocatalytic activity. Angew. Chem. 2002, 114, 2935–2937. [Google Scholar] [CrossRef]
- Ohno, T.; Tokieda, K.; Higashida, S.; Matsumura, M. Synergism between rutile and anatase TiO2 particles in photocatalytic oxidation of naphthalene. Appl. Catal. A Gen. 2003, 244, 383–391. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Dillert, R.; Feldhoff, A.; Bahnemann, D.W. Direct synthesis of photocatalytically active rutile TiO2 nanorods partly decorated with anatase nanoparticles. J. Phys. Chem. C 2010, 114, 4909–4915. [Google Scholar] [CrossRef]
- Tan, L.-L.; Ong, W.-J.; Chai, S.-P.; Mohamed, A.R. Visible-light-activated oxygen-rich TiO2 as next generation photocatalyst: Importance of annealing temperature on the photoactivity toward reduction of carbon dioxide. Chem. Eng. J. 2016, 283, 1254–1263. [Google Scholar] [CrossRef]
- Litter, M.I.; Román, E.S.; Grela, T.L.M.A.; Meichtry, J.M.; Rodríguez, H.B. Sensitization of TiO2 by dyes: A way to extend the range of photocatalytic activity of TiO2 to the visible region. In Visible Light-Active Photocatalysis: Nanostructured Catalyst Design, Mechanism and Applications; Wiley VCH: Weinheim, Germany, 2018; pp. 253–282. [Google Scholar]
- Nie, R.; Ma, W.; Dong, Y.; Xu, Y.; Wang, J.; Wang, J.; Jing, H. Artificial photosynthesis of methanol by Mn:CdS and CdSeTe quantum dot cosensitized titania photocathode in imine-based ionic liquid aqueous solution. ChemCatChem 2018, 10, 3342–3350. [Google Scholar] [CrossRef]
- Uner, D.; Oymak, M.M.; Ipek, B. CO2 utilisation by photocatalytic conversion to methane and methanol. Int. J. Glob. Warm. 2011, 3, 142. [Google Scholar] [CrossRef]
- Kaneco, S.; Kurimoto, H.; Ohta, K.; Mizuno, T.; Saji, A. Photocatalytic reduction of CO2 using TiO2 powders in liquid CO2 medium. J. Photochem. Photobiol. A Chem. 1997, 109, 59–63. [Google Scholar] [CrossRef]
- Anpo, M.; Yamashita, H.; Lichihashi, Y.; Fujii, Y.; Honda, M. Photocatalytic reduction of CO2 with H2O on titanium oxides anchored within micropores of zeolites: Effects of the structure of the active sites and the addition of Pt. J. Phys. Chem. B. 1997, 101, 2632–2636. [Google Scholar] [CrossRef]
- Adachi, K.; Ohta, K.; Mizuno, T. Photocatalytic reduction of carbon dioxide to hydrocarbon using copper-loaded titanium dioxide. Sol. Energy 1994, 53, 187–190. [Google Scholar] [CrossRef]
- Dey, G.; Belapurkar, A.; Kishore, K. Photo-catalytic reduction of carbon dioxide to methane using TiO2 as suspension in water. J. Photochem. Photobiol. A Chem. 2004, 163, 503–508. [Google Scholar] [CrossRef]
- Kočí, K.; Obalová, L.; Matějová, L.; Plachá, D.; Lacný, Z.; Jirkovský, J.; Šolcová, O. Effect of TiO2 particle size on the photocatalytic reduction of CO2. Appl. Catal. B Environ. 2009, 89, 494–502. [Google Scholar] [CrossRef]
- Wang, W.-N.; An, W.-J.; Ramalingam, B.; Mukherjee, S.; Niedzwiedzki, D.; Gangopadhyay, S.; Biswas, P. Size and structure matter: Enhanced CO2 photoreduction efficiency by size-resolved ultrafine Pt nanoparticles on TiO2 single crystals. J. Am. Chem. Soc. 2012, 134, 11276–11281. [Google Scholar] [CrossRef]
- Fang, B.; Xing, Y.; Bonakdarpour, A.; Zhang, S.; Wilkinson, D.P. Hierarchical CuO–TiO2 hollow microspheres for highly efficient photodriven reduction of CO2 to CH4. ACS Sustain. Chem. Eng. 2015, 3, 2381–2388. [Google Scholar] [CrossRef]
- Yui, T.; Kan, A.; Saitoh, C.; Koike, K.; Ibusuki, T.; Ishitani, O. Photochemical reduction of CO2 using TiO2: Effects of organic adsorbates on TiO2 and deposition of Pd onto TiO2. ACS Appl. Mater. Interfaces 2011, 3, 2594–2600. [Google Scholar] [CrossRef]
- Collado, L.; Reynal, A.; Coronado, J.; Serrano, D.; Durrant, J.; O’Shea, V.A.D.L.P. Effect of Au surface plasmon nanoparticles on the selective CO2 photoreduction to CH4. Appl. Catal. B Environ. 2015, 178, 177–185. [Google Scholar] [CrossRef]
- Nishimura, A.; Ishida, N.; Tatematsu, D.; Hirota, M.; Koshio, A.; Kokai, F.; Hu, E. Effect of Fe loading condition and reductants on CO2 reduction performance with Fe/TiO2 photocatalyst. Int. J. Photoenergy 2017, 2017, 1625274. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Liu, M.; Yang, B.; Shu, W.; Shen, Q.; Liu, M.; Zhou, J. Enhanced photocatalytic performance toward CO2 hydrogenation over nanosized TiO2-loaded Pd under UV irradiation. J. Phys. Chem. C 2017, 121, 2923–2932. [Google Scholar] [CrossRef]
- Camarillo, R.; Tostón, S.; Martinez, F.; Jiménez, C.; Rincón, J. Enhancing the photocatalytic reduction of CO2 through engineering of catalysts with high pressure technology: Pd/TiO2 photocatalysts. J. Supercrit. Fluids 2017, 123, 18–27. [Google Scholar] [CrossRef]
- Koci, K.; Matějů, K.; Obalová, L.; Krejčíková, S.; Lacný, Z.; Plachá, D.; Čapek, L.; Hospodková, A.; Solcova, O. Effect of silver doping on the TiO2 for photocatalytic reduction of CO2. Appl. Catal. B Environ. 2010, 96, 239–244. [Google Scholar] [CrossRef]
- Tu, W.; Li, Y.; Kuai, L.; Zhou, Y.; Xu, Q.; Li, H.; Wang, X.; Xiao, M.; Zou, Z. Construction of unique two-dimensional MoS2–TiO2 hybrid nanojunctions: MoS2 as a promising cost-effective cocatalyst toward improved photocatalytic reduction of CO2 to methanol. Nanoscale 2017, 9, 9065–9070. [Google Scholar] [CrossRef]
- She, H.; Zhou, H.; Li, L.; Zhao, Z.; Jiang, M.; Huang, J.; Wang, L.; Wang, Q. Construction of a two-dimensional composite derived from TiO2 and SnS2 for enhanced photocatalytic reduction of CO2 into CH4. ACS Sustain. Chem. Eng. 2018, 7, 650–659. [Google Scholar] [CrossRef]
- Low, J.; Dai, B.; Tong, T.; Jiang, C.; Yu, J. In situ irradiated X-ray photoelectron spectroscopy investigation on a direct Z-scheme TiO2/CdS composite film photocatalyst. Adv. Mater. 2019, 31, 1802981. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Luo, D.; Li, J.; Huang, Y.; Li, H.; Fang, Y.; Xu, Y.; Zhu, L. Adsorption of CO2 on heterostructure CdS (Bi2S3)/TiO2 nanotube photocatalysts and their photocatalytic activities in the reduction of CO2 to methanol under visible light irradiation. Chem. Eng. J. 2012, 180, 151–158. [Google Scholar] [CrossRef]
- Yang, G.; Chen, D.; Ding, H.; Feng, J.; Zhang, J.Z.; Zhu, Y.; Hamid, S.; Bahnemann, D.W. Well-designed 3D ZnIn2S4 nanosheets/TiO2 nanobelts as direct Z-scheme photocatalysts for CO2 photoreduction into renewable hydrocarbon fuel with high efficiency. Appl. Catal. B Environ. 2017, 219, 611–618. [Google Scholar] [CrossRef]
- Takayama, T.; Sato, K.; Fujimura, T.; Kojima, Y.; Iwase, A.; Kudo, A. Photocatalytic CO2 reduction using water as an electron donor by a powdered Z-scheme system consisting of metal sulfide and an RGO–TiO2 composite. Faraday Discuss. 2017, 198, 397–407. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, C.; Zuo, G.; Xiao, W.; Dai, W.; Kong, J.; Xu, X.; Zhou, Y.; Xie, A.; Sun, C.; et al. 0D/2D Co3O4/TiO2 Z-Scheme heterojunction for boosted photocatalytic degradation and mechanism investigation. Appl. Catal. B Environ. 2020, 278, 119298. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2018, 48, 2109–2125. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wang, W.; Liu, D.; Zhu, Q.; Jia, J.; Tian, J.; Li, Z.; Wang, X.; Xing, Z. Oxygen vacancy-mediated sandwich-structural TiO2-x/ultrathin g-C3N4/TiO2-x direct Z-scheme heterojunction visible-light-driven photocatalyst for efficient removal of high toxic tetracycline antibiotics. J. Haz. Mat. 2020, 408, 124432. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fan, Z.; Zhang, Z.; Niu, W.; Li, C.; Yang, N.; Chen, B.; Zhang, H. Two-dimensional metal nanomaterials: Synthesis, properties, and applications. Chem. Rev. 2018, 118, 6409–6455. [Google Scholar] [CrossRef] [PubMed]
- Fajrina, N.; Tahir, M. 2D-montmorillonite-dispersed g-C3N4/TiO2 2D/0Dnanocomposite for enhanced photo-induced H2 evolution from glycerol-water mixture. Appl. Surf. Sci. 2019, 471, 1053–1064. [Google Scholar] [CrossRef]
- Yu, J.; Wang, S.; Low, J.; Xiao, W. Enhanced photocatalytic performance of direct Z-scheme gC3N4–TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys. Chem. Chem. Phys. 2013, 15, 16883–16890. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Feng, Y.; Li, D.; Han, X.; Liu, J. Efficient photocatalytic CO2 reduction by P–O linked g-C3N4/TiO2-nanotubes Z-scheme composites. Energy 2019, 178, 168–175. [Google Scholar] [CrossRef]
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrog. Energy 2019, 44, 540–577. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.S.R. Comparison of modification strategies towards enhanced charge carrier separation and photocatalytic degradation activity of metal oxide semiconductors (TiO2, WO3 and ZnO). Appl. Surf. Sci. 2017, 391, 124–148. [Google Scholar] [CrossRef]
- Raza, A.; Shen, H.; Haidry, A.A.; Sun, L.; Liu, R.; Cui, S. Studies of Z-scheme WO3-TiO2/Cu2ZnSnS4 ternary nanocomposite with enhanced CO2 photoreduction under visible light irradiation. J. CO2 Util. 2020, 37, 260–271. [Google Scholar] [CrossRef]
- Wei, Y.; Jiao, J.; Zhao, Z.; Zhong, W.; Li, J.; Liu, J.; Jiang, G.; Duan, A. 3D ordered macroporous TiO2-supported Pt@ CdS core–shell nanoparticles: Design, synthesis and efficient photocatalytic conversion of CO2 with water to methane. J. Mater. Chem. A 2015, 3, 11074–11085. [Google Scholar] [CrossRef]
- Li, J.; Dong, X.; Sun, Y.; Cen, W.; Dong, F. Facet-dependent interfacial charge separation and transfer in plasmonic photocatalysts. Appl. Catal. B Environ. 2018, 226, 269–277. [Google Scholar] [CrossRef]
- Wei, X.; Shao, C.; Li, X.; Lu, N.; Wang, K.; Zhang, Z.; Liu, Y. Facile in situ synthesis of plasmonic nanoparticles-decorated gC3N4/TiO2 heterojunction nanofibers and comparison study of their photosynergistic effects for efficient photocatalytic H2 evolution. Nanoscale 2016, 8, 11034–11043. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, Y.; Xu, H.; Li, Y.; Wei, Y.; Liu, J.; Zhao, Z. Efficient Z-scheme photocatalysts of ultrathin g-C3N4-wrapped Au/TiO2-nanocrystals for enhanced visible-light-driven conversion of CO2 with H2O. Appl. Catal. B Environ. 2020, 263, 118314. [Google Scholar] [CrossRef]
- Chen, X.; Jin, F. Photocatalytic reduction of carbon dioxide by titanium oxide-based semiconductors to produce fuels. Front. Energy 2019, 13, 207–220. [Google Scholar] [CrossRef]
- Meng, X.; Wang, T.; Liu, L.; Ouyang, S.; Li, P.; Hu, H.; Kako, T.; Iwai, H.; Tanaka, A.; Ye, J. Photothermal conversion of CO2 into CH4 with H2 over Group VIII nanocatalysts: An alternative approach for solar fuel production. Angew. Chem. Int. Ed. 2014, 53, 11478–11482. [Google Scholar] [CrossRef] [PubMed]
- Vyas, V.S.; Lau, V.W.-H.; Lotsch, B.V. Soft photocatalysis: Organic polymers for solar fuel production. Chem. Mater. 2016, 28, 5191–5204. [Google Scholar] [CrossRef]
- Ghasimi, S. Conjugated Porous Polymers for Visible-light Photocatalysis. Ph.D. Thesis, Johannes Gutenberg-Universität Mainz, Mainz, Germany, 2016. [Google Scholar]
- Hou, J.; Jiang, T.; Wang, X.; Zhang, G.; Zou, J.; Cao, C. Variable dimensional structure and interface design of g-C3N4/BiOI composites with oxygen vacancy for improving visible-light photocatalytic properties. J. Clean. Prod. 2021, 287, 125072. [Google Scholar] [CrossRef]
- Apaydin, D.H.; Tordin, E.; Portenkirchner, E.; Aufischer, G.; Schlager, S.; Weichselbaumer, M.; Oppelt, K.; Sariciftci, N.S. Photoelectrochemical reduction of CO2Using third-generation conjugated polymers. ChemistrySelect 2016, 1, 1156–1162. [Google Scholar] [CrossRef]
- Rosen, M.A. Environmental sustainability tools in the biofuel industry. Biofuel Res. J. 2018, 5, 751–752. [Google Scholar] [CrossRef] [Green Version]
























Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, Z.U.; Bilal, M.; Hou, J.; Butt, F.K.; Ahmad, J.; Ali, S.; Hussain, A. Photocatalytic CO2 Reduction Using TiO2-Based Photocatalysts and TiO2 Z-Scheme Heterojunction Composites: A Review. Molecules 2022, 27, 2069. https://doi.org/10.3390/molecules27072069
Rehman ZU, Bilal M, Hou J, Butt FK, Ahmad J, Ali S, Hussain A. Photocatalytic CO2 Reduction Using TiO2-Based Photocatalysts and TiO2 Z-Scheme Heterojunction Composites: A Review. Molecules. 2022; 27(7):2069. https://doi.org/10.3390/molecules27072069
Chicago/Turabian StyleRehman, Zia Ur, Muhammad Bilal, Jianhua Hou, Faheem K. Butt, Junaid Ahmad, Saif Ali, and Asif Hussain. 2022. "Photocatalytic CO2 Reduction Using TiO2-Based Photocatalysts and TiO2 Z-Scheme Heterojunction Composites: A Review" Molecules 27, no. 7: 2069. https://doi.org/10.3390/molecules27072069
APA StyleRehman, Z. U., Bilal, M., Hou, J., Butt, F. K., Ahmad, J., Ali, S., & Hussain, A. (2022). Photocatalytic CO2 Reduction Using TiO2-Based Photocatalysts and TiO2 Z-Scheme Heterojunction Composites: A Review. Molecules, 27(7), 2069. https://doi.org/10.3390/molecules27072069