A Review on Regenerating Materials from Spent Lithium-Ion Batteries
Abstract
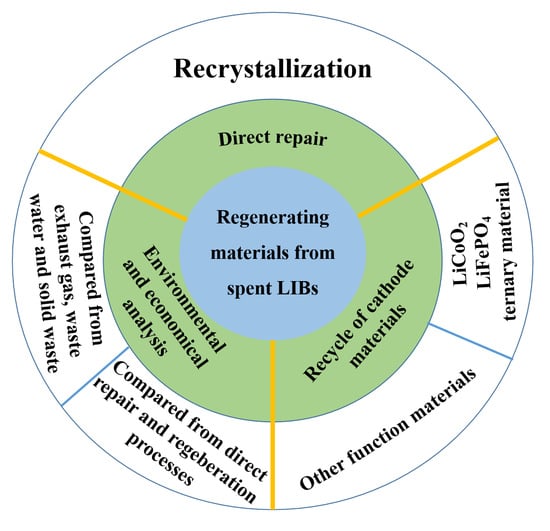
:1. Introduction
2. Technologies for Material Regeneration
2.1. Regeneration of Cathode Materials
2.1.1. Direct Repair
2.1.2. Materials Regeneration
- (1)
- Cathode Material Regeneration for LIBs
- (2)
- Other Function Materials

3. Environmental and Economic Analysis
3.1. Environmental Impact
3.2. Economic Aspects
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Lithium-ion batteries | LIBs |
| Composition annual growth rate | CAGR |
| Active discharge time | ADT |
| N-Methylpyrrolidine | NMP |
| Methylene blue | MB |
| Volatile organic chemicals | VOCs |
| Iron hydroxyl phosphate composites | FPOH |
| Li1.6Mn1.6−xFexO4 | LMO |
References
- Huang, B.; Pan, Z.; Su, X.; An, L. Recycling of Lithium-Ion Batteries: Recent Advances and Perspectives. J. Power Sources 2018, 399, 274–286. [Google Scholar] [CrossRef]
- Liao, Z.; Zhang, S.; Li, K.; Zhang, G.; Habetler, T.G. Review Article A Survey of Methods for Monitoring and Detecting Thermal Runaway of Lithium-Ion Batteries. J. Power Sources 2020, 436, 226879. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Z.; Lin, X.; Zhang, Y.; He, Y.; Cao, H.; Sun, Z. A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries. Engineering 2018, 4, 361–370. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Xu, Z. Generation and Detection of Metal Ions and Volatile Organic Compounds (VOCs) Emissions from the Pretreatment Processes for Recycling Spent Lithium-Ion Batteries. Waste Manag. 2016, 52, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lei, S.; Song, S.; Sun, W.; Wang, L. Stepwise Recycling of Valuable Metals from Ni-Rich Cathode Material of Spent Lithium-Ion Batteries. Waste Manag. 2020, 102, 131–138. [Google Scholar] [CrossRef]
- Lv, W.; Wang, Z.; Cao, H.; Sun, Y.; Zhang, Y.; Sun, Z. A Critical Review and Analysis on the Recycling of Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 1504–1521. [Google Scholar] [CrossRef]
- Chen, P.; Sun, Y.; Yang, L.; Xu, R.; Luo, Y.; Wang, X.; Cao, J.; Wang, J. Utilization of Metallurgy—Beneficiation Combination Strategy to Decrease TiO2 in Titanomagnetite Concentrate before Smelting. Minerals 2021, 11, 1419. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, Z.; Xue, F.; Yang, B.; Guo, G.; Qiu, J. A More Simple and Efficient Process for Recovery of Cobalt and Lithium from Spent Lithium-Ion Batteries with Citric Acid. Sep. Purif. Technol. 2019, 215, 398–402. [Google Scholar] [CrossRef]
- Yao, L.P.; Zeng, Q.; Qi, T.; Li, J. An Environmentally Friendly Discharge Technology to Pretreat Spent Lithium-Ion Batteries. J. Clean. Prod. 2019, 245, 118820. [Google Scholar] [CrossRef]
- Al-Thyabat, S.; Nakamura, T.; Shibata, E.; Iizuka, A. Adaptation of Minerals Processing Operations for Lithium-Ion (LiBs) and Nickel Metal Hydride (NiMH) Batteries Recycling: Critical Review. Miner. Eng. 2013, 45, 4–17. [Google Scholar] [CrossRef]
- Song, D.; Wang, X.; Nie, H.; Shi, H.; Wang, D.; Guo, F.; Shi, X.; Zhang, L. Heat Treatment of LiCoO2 Recovered from Cathode Scraps with Solvent Method. J. Power Sources 2014, 249, 137–141. [Google Scholar] [CrossRef]
- Wang, M.; Tan, Q.; Liu, L.; Li, J. A Low-Toxicity and High-Efficiency Deep Eutectic Solvent for the Separation of Aluminum Foil and Cathode Materials from Spent Lithium-Ion Batteries. J. Hazard. Mater. 2019, 380, 120846. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhu, J.; Qiu, R.; Ruan, J.; Qiu, R. A Cleaner and Energy-Saving Technology of Vacuum Step-by-Step Reduction for Recovering Cobalt and Nickel from Spent Lithium-Ion Batteries. J. Clean. Prod. 2019, 229, 1148–1157. [Google Scholar] [CrossRef]
- Mayyas, A.; Steward, D.; Mann, M. The Case for Recycling: Overview and Challenges in the Material Supply Chain for Automotive Li-Ion Batteries. Sustain. Mater. Technol. 2018, 17, e00087. [Google Scholar] [CrossRef]
- Saneie, R.; Abdollahi, H.; Ghassa, S.; Azizi, D.; Chehreh Chelgani, S. Recovery of Copper and Aluminum from Spent Lithium-Ion Batteries by Froth Flotation: A Sustainable Approach. J. Sustain. Metall. 2022, 8, 386–397. [Google Scholar] [CrossRef]
- Liu, J.; Shi, H.; Hu, X.; Geng, Y.; Yang, L.; Shao, P.; Luo, X. Critical Strategies for Recycling Process of Graphite from Spent Lithium-Ion Batteries: A Review. Sci. Total Environ. 2022, 816, 151621. [Google Scholar] [CrossRef]
- Hou, H.; Li, D.; Liu, X.; Yao, Y.; Dai, Z.; Yu, C. Recovery of Waste Li Foils from Spent Experimental Li-Anode Coin Cells for LiFePO4/C Cathode. Sustain. Mater. Technol. 2018, 17, e00064. [Google Scholar] [CrossRef]
- Li, L.; Chen, R.J.; Zhang, X.X.; Wu, F.; Ge, J.; Xie, M. Preparation and Electrochemical Properties of Re-Synthesized LiCoO2 from Spent Lithium-Ion Batteries. Chin. Sci. Bull. 2012, 57, 4188–4194. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Li, J.; Zhou, T.; Yang, K.; Wei, S.; Tang, N.; Dang, N.; Li, H.; Qiu, X.; Chen, L. Nano Energy Toxicity, a Serious Concern of Thermal Runaway from Commercial Li-Ion. Nano Energy 2016, 27, 313–319. [Google Scholar] [CrossRef]
- Shuya, L.; Yang, C.; Xuefeng, C.; Wei, S.; Yaqing, W.; Yue, Y. Separation and Puri Fi Cation Technology Separation of Lithium and Transition Metals from Leachate of Spent Lithium- Ion Batteries by Solvent Extraction Method with Versatic 10. Sep. Purif. Technol. 2020, 250, 117258. [Google Scholar] [CrossRef]
- Lou, P.; Guan, M.; Wu, G.; Wu, J.; Yu, H.; Zhang, W.; Cheng, Q. Recycle Cathode Materials from Spent Lithium-Ion Batteries by an Innovative Method. Ionics 2022. Available online: https://doi.org/10.1007/s11581-022-04497-4 (accessed on 3 March 2022).
- Kriston, A.; Adanouj, I.; Ruiz, V.; Pfrang, A. Quantification and Simulation of Thermal Decomposition Reactions of Li-Ion Battery Materials by Simultaneous Thermal Analysis Coupled with Gas Analysis. J. Power Sources 2019, 435, 226774. [Google Scholar] [CrossRef]
- Liu, B.; Huang, Q.; Su, Y.; Sun, L.; Wu, T.; Wang, G.; Kelly, R.M.; Wu, F. Maleic, Glycolic and Acetoacetic Acids-Leaching for Recovery of Valuable Metals from Spent Lithium-Ion Batteries: Leaching Parameters, Thermodynamics and Kinetics. R. Soc. Open Sci. 2019, 6, 191061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Q.; Yue, H.; Wang, S.; Yang, S.; Lam, K.; Hou, X. Recycling and Crystal Regeneration of Commercial Used LiFePO4 Cathode Materials. Electro Acta 2019, 330, 135323. [Google Scholar] [CrossRef]
- Yang, H.; Deng, B.; Jing, X.; Li, W.; Wang, D. Direct Recovery of Degraded LiCoO2 Cathode Material from Spent Lithium-Ion Batteries: Efficient Impurity Removal toward Practical Applications. Waste Manag. 2021, 129, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhong, S.; Xiong, D.; Chen, H. Synthesis and Electrochemical Performances of LiCoO2 Recycled from the Incisors Bound of Li-Ion Batteries. Rare Met. 2009, 28, 328–332. [Google Scholar] [CrossRef]
- Zhang, Z.; He, W.; Li, G.; Xia, J.; Hu, H.; Huang, J. Renovation of LiCoO2 Crystal Structure from Spent Lithium Ion Batteries by Ultrasonic Hydrothermal Reaction. Res. Chem. Intermed. 2015, 41, 3367–3373. [Google Scholar] [CrossRef]
- Kim, D.S.; Sohn, J.S.; Lee, C.K.; Lee, J.H.; Han, K.S.; Lee, Y.-I. Simultaneous Separation and Renovation of Lithium Cobalt Oxide from the Cathode of Spent Lithium Ion Rechargeable Batteries. J. Power Sources 2004, 132, 145–149. [Google Scholar] [CrossRef]
- Sun, Q.; Li, X.; Zhang, H.; Song, D.; Shi, X.; Song, J.; Li, C.; Zhang, L. Resynthesizing LiFePO4/C Materials from the Recycled Cathode via a Green Full-Solid Route. Alloys Compd. 2020, 818, 153292. [Google Scholar] [CrossRef]
- Chen, J.; Li, Q.; Song, J.; Song, D.; Zhang, L.; Shi, X. Environmentally Friendly Recycling and Effective Repairing of Cathode Powders from Spent LiFePO4 Batteries. Green Chem. 2016, 2500–2506. [Google Scholar] [CrossRef]
- Online, V.A.; Song, X.; Hu, T.; Liang, C.; Long, H.L.; Zhou, L.; Song, W.; You, L.; Wu, Z.S.; Liu, J.W. Direct Regeneration of Cathode Materials from Spent Lithium Iron Phosphate Batteries Using a Solid Phase Sintering Method. RSC Adv. 2017, 4783–4790. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Lin, X.Y.; Tang, Y.; Wang, Q.; Leung, M.K.H.; Lu, X.Y. Recycling LiCoO2 with Methanesulfonic Acid for Regeneration of Lithium-Ion Battery Electrode Materials. J. Power Sources 2019, 436, 226828. [Google Scholar] [CrossRef]
- Song, Y.; Xie, B.; Song, S.; Lei, S.; Sun, W.; Xu, R.; Yang, Y. Regeneration of LiFePO4 from Spent Lithium-Ion Batteries via a Facile Process Featuring Acid Leaching and Hydrothermal Synthesis. Green Chem. 2021, 23, 3963–3971. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, S.; He, Y. Lithium Recycling and Cathode Material Regeneration from Acid Leach Liquor of Spent Lithium-Ion Battery via Facile Co-Extraction and Co-Precipitation Processes. Waste Manag. 2017, 64, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.J.; Li, X.Q.; Li, X.H.; Wang, Z.X.; Peng, W.X.; Sun, Q.M.; Xie, J. Preparation and Electrochemical Properties of Co3O4/Graphite Composites as Anodes of Lithium Ion Batteries. J. Cent. South Univ. Technol. 2010, 17, 498–503. [Google Scholar] [CrossRef]
- Guo, M.; Li, K.; Liu, L.; Zhang, H.; Guo, W.; Hu, X.; Meng, X.; Jia, J.; Sun, T. Manganese-Based Multi-Oxide Derived from Spent Ternary Lithium-Ions Batteries as High-Efficient Catalyst for VOCs Oxidation. J. Hazard. Mater. 2019, 380, 120905. [Google Scholar] [CrossRef]
- Shen, B.; Zhou, P.; Chen, S.; Yuan, H.; Zhu, N.; Sun, T.; Lou, Z. Manganese-Based Catalysts Recovered from Spent Ternary Lithium-Ion Batteries and Its Catalytic Activity Enhanced by a Mechanical Method. J. Clean. Prod. 2019, 213, 1346–1352. [Google Scholar] [CrossRef]
- Guo, M.; Li, K.; Liu, L.; Zhang, H.; Hu, X.; Min, X.; Jia, J.; Sun, T. Resource Utilization of Spent Ternary Lithium-Ions Batteries: Synthesis of Highly Active Manganese-Based Perovskite Catalyst for Toluene Oxidation. J. Taiwan Inst. Chem. Eng. 2019, 102, 268–275. [Google Scholar] [CrossRef]
- Xu, L.; Chen, C.; Huo, J.B.; Chen, X.; Yang, J.C.E.; Fu, M.L. Iron Hydroxyphosphate Composites Derived from Waste Lithium-Ion Batteries for Lead Adsorption and Fenton-like Catalytic Degradation of Methylene Blue. Environ. Technol. Innov. 2019, 16, 100504. [Google Scholar] [CrossRef]
- Shen, J.; Pierce, J.P.; Plummer, E.W.; Kirschner, J. The Effect of Spatial Confinement on Magnetism: Films, Stripes and Dots of Fe on Cu(111). J. Phys. Condens. Matter 2003, 15, R1. [Google Scholar] [CrossRef]
- Xi, G.; Xu, H.; Yao, L. Study on Preparation of NiCo Ferrite Using Spent Lithium-Ion and Nickel-Metal Hydride Batteries. Sep. Purif. Technol. 2015, 145, 50–55. [Google Scholar] [CrossRef]
- Rocha, A.K.S.; Magnago, L.B.; Santos, J.J.; Leal, V.M.; Marins, A.A.L.; Pegoretti, V.C.B.; Ferreira, S.A.D.; Lelis, M.F.F.; Freitas, M.B.J.G. Copper Ferrite Synthesis from Spent Li-Ion Batteries for Multifunctional Application as Catalyst in Photo Fenton Process and as Electrochemical Pseudocapacitor. Mater. Res. Bull. 2019, 113, 231–240. [Google Scholar] [CrossRef]
- Nie, X.-J.; Xi, X.-T.; Yang, Y.; Ning, Q.-L.; Guo, J.-Z.; Wang, M.-Y.; Gu, Z.-Y.; Wu, X.-L. Recycled LiMn2O4 from the Spent Lithium Ion Batteries as Cathode Material for Sodium Ion Batteries: Electrochemical Properties, Structural Evolution and Electrode Kinetics. Electrochim. Acta 2019, 320, 134626. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.; Fu, Y.; Huang, H.; Zhong, Z.; Wang, Y. Recovery of Fe, Mn, Ni and Co in Sulfuric Acid Leaching Liquor of Spent Lithium Ion Batteries for Synthesis of Lithium Ion-Sieve and NixCoyMn1−x−y(OH)2. Hydrometallurgy 2019, 190, 105190. [Google Scholar] [CrossRef]
- Cheng, Y.; Guo, G.; Cheng, X.; Liu, M.; Ji, J. Synthesis and Research of MnO2–NiCo2O4 Anode Material from Spent LiNi0.6Co0.2Mn0.2O2 Cathodes. Ionics 2022, 28, 1647–1656. [Google Scholar] [CrossRef]
- Almehmadi, F.A.; Alqaed, S.; Mustafa, J.; Jamil, B.; Sharifpur, M.; Cheraghian, G. Combining an Active Method and a Passive Method in Cooling Lithium-Ion Batteries and Using the Generated Heat in Heating a Residential Unit. J. Energy Storage 2022, 49, 104181. [Google Scholar] [CrossRef]
- Tharumalingam, E.; Dusseault, M.B.; Fraser, R. Study of Energy Storage Systems and Environmental Challenges of Batteries. Renew. Sustain. Energy Rev. 2019, 104, 192–208. [Google Scholar] [CrossRef]
- Andrey, W.G.; Sebastian, S.; René, P.; Gernot, V.; Helmar, W.; Christoph, S.; Gisela, F. Thermal runaway of commercial 18650 Li-ion batteries with LFP and NCA cathodes-impact of state of charge and overcharge. RSC Adv. 2015, 5, 57171. [Google Scholar] [CrossRef] [Green Version]
- Busche, M.R.; Adelhelm, P.; Sommer, H.; Schneider, H.; Leitner, K.; Janek, J. Systematical Electrochemical Study on the Parasitic Shuttle-Effect in Lithium-Sulfur-Cells at Different Temperatures and Different Rates. J. Power Sources 2014, 259, 289–299. [Google Scholar] [CrossRef]
- Larsson, F.; Bertilsson, S.; Furlani, M.; Albinsson, I.; Mellander, B. Gas Explosions and Thermal Runaways during External Heating Abuse of Commercial Lithium-Ion Graphite-LiCoO2 Cells at different Levels of Ageing. J. Power Sources 2018, 373, 220–231. [Google Scholar] [CrossRef]
- Diaz, F.; Wang, Y.; Weyhe, R.; Friedrich, B. Gas Generation Measurement and Evaluation during Mechanical Processing and Thermal Treatment of Spent Li-Ion Batteries. Waste Manag. 2019, 84, 102–111. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, N.; Jie, Y.; Hu, F.; Li, Y.; Wilson, B.P.; Xi, Y.; Lai, Y.; Yang, S. Toxicity Identification and Evolution Mechanism of Thermolysis-Driven Gas Emissions from Cathodes of Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 18228–18235. [Google Scholar] [CrossRef]
- Ruffino, B.; Zanetti, M.C.; Marini, P. A Mechanical Pre-Treatment Process for the Valorization of Useful Fractions from Spent Batteries. Resour. Conserv. Recycl. 2011, 55, 309–315. [Google Scholar] [CrossRef]
- Yang, Y.; Song, S.; Jiang, F.; Zhou, J.; Sun, W. Short Process for Regenerating Mn-Rich Cathode Material with High Voltage from Mixed-Type Spent Cathode Materials via a Facile Approach. J. Clean. Prod. 2018, 186, 123–130. [Google Scholar] [CrossRef]
- Chen, Y.G.; Wang, C.G.; Zhang, X.Y.; Xie, D.M.; Wang, R.S. Lithium Salts of Heteropolyacid as the Electrolyte of Lithium-Ion Battery. Synth. Met. 2003, 135, 225–226. [Google Scholar] [CrossRef]
- Liu, W.; Zhong, X.; Han, J.; Qin, W.; Liu, T.; Zhao, C.; Chang, Z. Kinetic Study and Pyrolysis Behaviors of Spent LiFePO4 Batteries. ACS Sustain. Chem. Eng. 2018, 7, 1289–1299. [Google Scholar] [CrossRef]
- Liu, C.; Lin, J.; Cao, H.; Zhang, Y.; Sun, Z. Recycling of Spent Lithium-Ion Batteries in View of Lithium Recovery: A Critical Review. J. Clean. Prod. 2019, 228, 801–813. [Google Scholar] [CrossRef]
- Peng, F.; Mu, D.; Li, R.; Liu, Y.; Ji, Y.; Dai, C.; Ding, F. Impurity Removal with Highly Selective and efficient. RSC Adv. 2019, 9, 21922–21930. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Qiu, J.; Yu, M.; Jin, C.; Yang, B.; Guo, G. Performance of Al-Doped LiNi1/3Co1/3Mn1/3O2 Synthesized from Spent Lithium Ion Batteries by Sol-Gel Method. Vacuum 2020, 172, 109105. [Google Scholar] [CrossRef]
- Wu, C.; Li, B.; Yuan, C.; Ni, S.; Li, L. Recycling Valuable Metals from Spent Lithium-Ion Batteries by Ammonium Sulfite-Reduction Ammonia Leaching. Waste Manag. 2019, 93, 153–161. [Google Scholar] [CrossRef]
- Yang, L.; Ren, F.; Feng, Q.; Xu, G.; Li, X.; Li, Y.; Zhao, E.; Ma, J.; Fan, S. Effect of Cu Doping on the Structural and Electrochemical Performance of LiNi1/3Co1/3Mn1/3O2 Cathode Materials. J. Electron. Mater. 2018, 47, 3996–4002. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Z.; Chen, L. Comparison of Structure and Electrochemistry of Al- and Fe-Doped LiNi1/3Co1/3Mn1/3O2. Electrochem. Acta 2006, 51, 4199–4203. [Google Scholar] [CrossRef]
- Sa, Q.; Heelan, J.A.; Lu, Y.; Apelian, D.; Wang, Y. Copper Impurity Effects on LiNi1/3Mn1/3Co1/3O2 Cathode Material. ACS Appl. Mater. Interfaces 2015, 7, 20585–20590. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, L.; Xi, X.; Nie, Z.; Zhang, Y.; Wen, X.; Lyu, Z. Regeneration and Characterization of LiNi0.8Co0.15Al0.05O2 Cathode Material from Spent Power Lithium-Ion Batteries. Waste Manag. 2019, 95, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Yang, Y.; Zhang, T.; Wu, X.; Sun, W.; Yi, L. A Green and Facile Approach for Regeneration of Graphite from Spent Lithium Ion Battery. J. Clean. Prod. 2020, 277, 123585. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling Lithium-Ion Batteries from Electric Vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, P.; Dai, Q.; Gao, H.; Lu, J.; Chen, Z.; Xu, P.; Dai, Q.; Gao, H.; Liu, H.; Zhang, M.; et al. Report Efficient Direct Recycling of Lithium-Ion Battery Cathodes by Targeted Healing Efficient Direct Recycling of Lithium-Ion Battery Cathodes by Targeted Healing. Joule 2020, 4, 2609–2626. [Google Scholar] [CrossRef]
- Alqaed, S.; Almehmadi, F.A.; Mustafa, J.; Husain, S.; Cheraghian, G. Effect of Nano Phase Change Materials on the Cooling Process of a Triangular Lithium Battery Pack. J. Energy Storage 2022, 51, 104326. [Google Scholar] [CrossRef]








| The Number of Documents | 78 |
|---|---|
| Documents in China | 60 |
| Documents in Spain | 2 |
| Documents in USA | 4 |
| Documents in UK | 1 |
| Documents in Germany | 2 |
| Documents in Italy | 1 |
| Documents in Sweden | 1 |
| Documents in Austria | 1 |
| Documents in Canada | 1 |
| Documents in Brazil | 1 |
| Documents in South Korea | 1 |
| Documents in the Netherlands | 1 |
| Documents in Iran | 1 |
| Documents in Japan | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, R.; Xu, W.; Wang, J.; Liu, F.; Sun, W.; Yang, Y. A Review on Regenerating Materials from Spent Lithium-Ion Batteries. Molecules 2022, 27, 2285. https://doi.org/10.3390/molecules27072285
Xu R, Xu W, Wang J, Liu F, Sun W, Yang Y. A Review on Regenerating Materials from Spent Lithium-Ion Batteries. Molecules. 2022; 27(7):2285. https://doi.org/10.3390/molecules27072285
Chicago/Turabian StyleXu, Rui, Wei Xu, Jinggang Wang, Fengmei Liu, Wei Sun, and Yue Yang. 2022. "A Review on Regenerating Materials from Spent Lithium-Ion Batteries" Molecules 27, no. 7: 2285. https://doi.org/10.3390/molecules27072285
APA StyleXu, R., Xu, W., Wang, J., Liu, F., Sun, W., & Yang, Y. (2022). A Review on Regenerating Materials from Spent Lithium-Ion Batteries. Molecules, 27(7), 2285. https://doi.org/10.3390/molecules27072285