Activated Carbon/Pectin Composite Enterosorbent for Human Protection from Intoxication with Xenobiotics Pb(II) and Sodium Diclofenac
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthetic Approach (Methodology) Insight
2.2. Low-Temperature Nitrogen Adsorption Studies of Rice Husk Derived Activated Carbon (AC-RH)
2.3. FTIR Spectroscopy and Degree of Esterification of Pectins
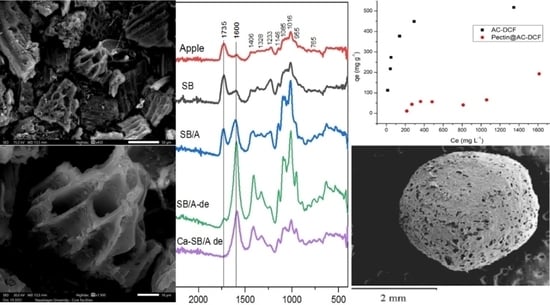
2.3.1. FTIR Spectroscopy Qualitative Analysis
2.3.2. FTIR-Spectroscopy Semi-Quantitative Analysis of Pectin’s Degree of Esterification
Degree of Esterification (DEst)
2.4. Surface Structure Morphology and the Elemental Content of the Composite Enterosorbent Components According to SEM/EDS-Analysis
2.5. Batch Adsorption Studies
2.5.1. Adsorption Kinetics
2.5.2. Adsorption Isotherms/Equilibrium Studies
3. Materials and Methods
3.1. Materials
3.2. AC-RH@Pectin-Based Core/Shell Granular Composite Synthesis
3.2.1. Activation of Carbonized Rice Husk with Potassium Hydroxide
3.2.2. Isolation of Pectin from Sugar Beet and Apple Pulp by Acidic Extraction
3.2.3. De-Esterification of Pectins by Alkaline Hydrolysis
3.2.4. Coating of Spherized De-Esterified Pectin Beads with Micronized AC-RH
3.3. Physicochemical Characterization of Adsorbents
3.3.1. Low-Temperature Nitrogen Adsorption
3.3.2. FTIR-Spectroscopy of Pectins
3.3.3. Scanning Electron Microscopy and EDS-Analysis
3.4. Batch Adsorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Belyakov, N.A.E. Enterosorption; Centre for Sorption Technologies: Leningrad, Russia, 1991. (In Russian) [Google Scholar]
- Vanitha, T.; Khan, M. Role of Pectin in Food Processing and Food Packaging; IntechOpen Ltd.: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Walter, R.H. The Chemistry and Technology of Pectin; Academic Press: Cambridge, MA, USA, 1991. [Google Scholar]
- Celus, M.; Kyomugasho, C.; van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Influence of pectin structural properties on interactions with divalent cations and its associated functionalities. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1576–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Liang, R.; Dai, T.; Chen, J.; Shuai, X.; Liu, C. Pectin-Based adsorbents for heavy metal ions: A review. Trends Food Sci. Technol. 2019, 91, 319–329. [Google Scholar] [CrossRef]
- Shao, Z.; Lu, J.; Ding, J.; Fan, F.; Sun, X.; Li, P.; Fang, Y.; Hu, Q. Novel green chitosan-pectin gel beads for the removal of Cu(II), Cd(II), Hg(II) and Pb(II) from aqueous solution. Int. J. Biol. Macromol. 2021, 176, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.E.; Mohamed, A.K. Novel derived pectin hydrogel from mandarin peel based metal-organic frameworks composite for enhanced Cr(VI) and Pb(II) ions removal. Int. J. Biol. Macromol. 2020, 164, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Koksharov, S.A.; Aleeva, S.V.; Lepilova, O.V. Description of adsorption interactions of lead ions with functional groups of pectin-containing substances. J. Mol. Liq. 2019, 283, 606–616. [Google Scholar] [CrossRef]
- Khotimchenko, M.Y.; Kolenchenko, E.A. Efficiency of low-esterified pectin in toxic damage to the liver inflicted by lead treatment. Bull. Exp. Biol. Med. 2007, 144, 60–62. [Google Scholar] [CrossRef]
- Trakhtenberg, I.M.; Litenko, V.A.; Derevyago, I.B.; Demchenko, P.I.; Mikhailovsky, S.V. Pectin-Containing sorbents for protection of the organism against Radionuclids and heavy metals. Likars’ka Sprav. 1992, 5, 29–33. [Google Scholar]
- FAQs About Rare Diseases|Genetic and Rare Diseases Information Center (GARD)—An NCATS Program. Available online: https://rarediseases.info.nih.gov/diseases/pages/31/faqs-about-rare-diseases (accessed on 2 March 2022).
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [Green Version]
- Bessone, F. Non-Steroidal anti-inflammatory drugs: What is the actual risk of liver damage? World J. Gastroenterol. 2010, 16, 5651–5661. [Google Scholar] [CrossRef]
- Björnsson, E.; Olsson, R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology 2005, 42, 481–489. [Google Scholar] [CrossRef]
- Kalderis, D.; Bethanis, S.; Paraskeva, P.; Diamadopoulos, E. Production of activated carbon from bagasse and rice husk by a single-stage chemical activation method at low retention times. Bioresour. Technol. 2008, 99, 6809–6816. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, X.; Guo, Y.; Rong, C.; Wang, L.; Qu, Y.; Ma, X.; Wang, Z. A new method of comprehensive utilization of rice husk. J. Hazard. Mater. 2011, 186, 2151–2156. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, E.; Nordin, A.; Rao, A.N. Overview of combustion and gasification of rice husk in fluidized bed reactors. Biomass Bioenergy 1998, 14, 533–546. [Google Scholar] [CrossRef]
- Jandosov, J.; Shikina, N.; Bijsenbayev, M.; Shamalov, M.; Ismagilov, Z.; Mansurov, Z. Evaluation of Synthetic conditions for H3PO4 chemically activated rice husk and preparation of honeycomb monoliths. Eurasian Chem.-Technol. J. 2009, 11, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Neethirajan, S.; Gordon, R.; Wang, L. Potential of silica bodies (Phytoliths) for nanotechnology. Trends Biotechnol. 2009, 27, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Jandosov, J.M.; Mansurov, Z.A.; Biisenbayev, M.A.; Ismagilov, Z.R.; Shikina, N.V.; Ismagilov, I.Z.; Konuspayev, S.R.; Shaymardan, M. Mesoporous carbon-based rhodium catalysts for benzene hydrogenation. Eurasian Chem.-Technol. J. 2012, 14, 37–40. [Google Scholar] [CrossRef] [Green Version]
- Jandosov, J.; Mansurov, Z.; Biisenbayev, M.; Kerimkulova, A.; Ismagilov, Z.; Shikina, N.; Ismagilov, I.; Andrievskaya, I. Mesoporous composite materials from Acivated rice husk carbon and montmorillonite. Eurasian Chem.-Technol. J. 2011, 13, 105–113. [Google Scholar] [CrossRef]
- Atamanov, M.; Amrousse, R.; Jandosov, J.; Hori, K.; Kerimkulova, A.; Chenchik, D.; Kolesnikov, B. Combustion characteristics of HAN-Based green propellant assisted with Nanoporous active carbons. Eurasian Chem.-Technol. J. 2017, 19, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Dias, D.; Don, D.; Jandosov, J.; Bernardo, M.; Pinto, F.; Fonseca, I.; Sanches, A.; Caetano, P.S.; Lyubchyk, S.; Lapa, N. Highly efficient porous carbons for the removal of W(VI) oxyanion from wastewaters. J. Hazard. Mater. 2021, 412, 125201. [Google Scholar] [CrossRef]
- Mansurov, Z.; Jandosov, J.; Kerimkulova, A.; Azat, S.; Zhubanova, A.; Digel, I.; Savistkaya, I.; Akimbekov, N.; Kistaubaeva, A.; Kistaubaeva, A. Nanostructured carbon materials for biomedical use. Eurasian Chem.-Technol. J. 2013, 15, 209–217. [Google Scholar] [CrossRef]
- Jandosov, J.; Mikhalovska, L.; Howell, C.; Chenchik, D.; Kosher, B.; Lyubchik, S.K.; Silvestre-Albero, J.; Ablaikhanova, N.; Srailova, G.; Tuleukhanov, S.; et al. Synthesis, morphostructure, surface chemistry and preclinical studies of nanoporous rice husk-derived Biochars for gastrointestinal detoxification. Eurasian Chem.-Technol. J. 2017, 19, 303–313. [Google Scholar] [CrossRef]
- Tekutskaya, E.E. Detoxical aspects of nutritional therapy using natural Enterosorbents on the basis of Pectins. Russ. Open Med. J. 2013, 2, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Khotimchenko, M.; Serguschenko, I.; Khotimchenko, Y. Lead absorption and excretion in rats given insoluble salts of pectin and alginate. Int. J. Toxicol. 2006, 25, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Kodoth, A.; Badalamoole, V. Pectin based ZnO nanocomposite hydrogel: Evaluation as Adsorbent for divalent metal ions from aqueous solutions. Nanotechnology 2017, 107, 47326–47331. [Google Scholar]
- Wang, X.; Li, Y.; Dai, T.; He, X.; Chen, M.; Liu, C.; Liang, R.; Chen, J. Preparation of Pectin/Poly(m-Phenylenediamine) Microsphere and its application for Pb2+ removal. Carbohydr. Polym. 2021, 260, 117811. [Google Scholar] [CrossRef]
- Savina, I.N.; Otero-Gonzalez, L.; Berillo, D.A. Chapter Macroporous Cryogel-Based Systems for Water Treatment Applications: Nanocomposite-Based Cryogels and Bacteria-Based Bioreactors. In Safety and Applications of Chemicals and Nanoparticles; Mohanan, P.V., Ed.; Cambridge Scholars Publishing Limited: Newcastle upon Tyne, UK, 2021. [Google Scholar]
- Araújo, L.D.C.B.; de Matos, H.K.; Facchi, D.P.; de Almeida, D.A.; Gonçalves, B.M.G.; Monteiro, J.P.; Martins, A.F.; Bonafé, E.G. Natural carbohydrate-based thermosensitive chitosan/pectin adsorbent for removal of Pb(II) from aqueous solutions. Int. J. Biol. Macromol. 2021, 193, 1813–1822. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Mata, Y.; Blázquez, M.L.; Ballester, A.; González, F.; Muñoz, J. Sugar-Beet pulp pectin gels as Biosorbent for heavy metals: Preparation and determination of biosorption and desorption characteristics. Chem. Eng. J. 2009, 150, 289–301. [Google Scholar] [CrossRef]
- Lefsih, K.; Delattre, C.; Pierre, G.; Michaud, P.; Aminabhavi, T.M.; Dahmoune, F.; Madani, K. Extraction, characterization and gelling behavior enhancement of Pectins from the cladodes of opuntia Ficus Indica. Int. J. Biol. Macromol. 2016, 82, 645–652. [Google Scholar] [CrossRef]
- Wang, F.; Du, C.; Chen, J.; Shi, L.; Li, H. A new method for determination of pectin content using spectrophotometry. Polymers 2021, 13, 2847. [Google Scholar] [CrossRef]
- Ouwerx, C.; Velings, N.; Mestdagh, M.M.; Axelos, M.A. Physico-Chemical Properties and rheology of alginate gel beads formed with various divalent cations. Polym. Gels Netw. 1998, 6, 393–408. [Google Scholar] [CrossRef]
- Schubert, J.; Riley, E.J.; Tyler, S.A. Combined effects in toxicology–a rapid systematic testing procedure: Cadmium, mercury, and lead. J. Toxicol. Environ. Health 1978, 4, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Hunter, L.J.; Wood, D.M.; Dargan, P.I. The Patterns of toxicity and management of acute nonsteroidal anti-inflammatory drug (NSAID) overdose. Open Access Emerg. Med. OAEM 2011, 3, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Fallingborg, J. Intraluminal PH of the human gastrointestinal tract. Dan. Med. Bull. 1999, 46, 183–196. [Google Scholar]
- Fernandez, M.E.; Ledesma, B.; Román, S.; Bonelli, P.R.; Cukierman, A.L. Development and Characterization of activated hydrochars from orange peels as potential adsorbents for emerging organic contaminants. Bioresour. Technol. 2015, 183, 221–228. [Google Scholar] [CrossRef]
- Saucier, C.; Adebayo, M.A.; Lima, E.C.; Cataluña, R.; Thue, P.S.; Prola, L.D.T.; Puchana-Rosero, M.J.; Machado, F.M.; Pavan, F.A.; Dotto, G.L. Microwave-Assisted activated carbon from cocoa shell as adsorbent for removal of sodium diclofenac and Nimesulide from aqueous effluents. J. Hazard. Mater. 2015, 289, 18–27. [Google Scholar] [CrossRef]
- Bernardo, M.; Rodrigues, S.; Lapa, N.; Matos, I.; Lemos, F.; Batista, M.K.S.; Carvalho, A.P.; Fonseca, I. High efficacy on diclofenac removal by activated carbon produced from potato peel waste. Int. J. Environ. Sci. Technol. 2016, 13, 1989–2000. [Google Scholar] [CrossRef]
- Sotelo, J.L.; Rodríguez, A.; Álvarez, S.; García, J. Removal of caffeine and diclofenac on activated carbon in fixed bed column. Chem. Eng. Res. Des. 2012, 90, 967–974. [Google Scholar] [CrossRef]
- Mao, N.; Huang, L.; Shuai, Q. Facile synthesis of porous carbon for the removal of diclofenac sodium from water. ACS Omega 2019, 4, 15051–15060. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Li, Y.; Shuai, X.; Liang, R.; Chen, J.; Liu, C. Pectin/Activated carbon-based porous microsphere for Pb2+ adsorption: Characterization and adsorption behaviour. Polymers 2021, 13, 2453. [Google Scholar] [CrossRef]
- Khotimchenko, M.; Kovalev, V.; Khotimchenko, Y. Equilibrium studies of sorption of Lead(II) ions by different pectin compounds. J. Hazard. Mater. 2007, 149, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Merkel, A.; Satayeva, A.; Cannon, F.; Howell, C.; Meikle, S.; László, K.; Inglezakis, V.; Jandosov, J.; Ray, S.; Mansurov, Z.; et al. Characterisation of activated carbons obtained from rice husk. Eurasian Chem.-Technol. J. 2016, 18, 299–304. [Google Scholar] [CrossRef] [Green Version]
- Jandosov, J.M.; Baimenov, A.Z.; Iklasova, A.S.; Sakipova, Z.B.; Sakenova, N.; László, K. Isolation and rheological properties of pectins derived from vegetable pulp. Vestn. KazNMU 2020, 4, 474–479. [Google Scholar]
- Haul, R.S.J.; Gregg, K.S.W. Sing: Adsorption, surface area and porosity. 2. auflage, Academic Press, London 1982. 303 Seiten, Preis: $49.50. Ber. Der Bunsenges. Für Phys. Chem. 1982, 86, 957. [Google Scholar] [CrossRef]
- Marques, M.; Löbenberg, R.; Almukainzi, M. Simulated biological fluids with possible application in dissolution testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Rafiee Taqanaki, E.; Heidari, R.; Monfared, M.; Tayebi, L.; Azadi, A.; Farjadian, F. EDTA-Modified mesoporous silica as supra adsorbent of copper ions with novel approach as an antidote agent in copper toxicity. Int. J. Nanomed. 2019, 14, 7781. [Google Scholar] [CrossRef] [Green Version]
- Baimenov, A.; Berillo, D.; Azat, S.; Nurgozhin, T.; Inglezakis, V. Removal of Cd2+ from water by use of super-macroporous Cryogels and comparison to commercial adsorbents. Polymers 2020, 12, 2405. [Google Scholar] [CrossRef]








| Sample Code | SB | Apple | SB/A | DE SB/A |
|---|---|---|---|---|
| -OH-group maximum wavenumber, cm−1 | 3380 | 3360 | 3330 | 3270 |
| DEst, % | 60.3 | 79.1 | 30.4 | 6.5 |
| Sample | Adsorbate | Pseudo-First Order | Pseudo-Second Order | ||||
|---|---|---|---|---|---|---|---|
| qecal (mg/g) | k1 (min−1) | R2 | qecal (mg/g) | k2 (g·mg−1·min−1) | R2 | ||
| AC-RH | Pb2+ | 3.14 | 2.38 × 10−3 | 0.982 | 38.8 | 4.904 × 10−3 | 0.999 |
| Pectin | Pb2+ | 11.10 | 3.05 × 10−3 | 0.899 | 77.88 | 1.475 × 10−3 | 0.999 |
| AC-RH@Pectin- | Pb2+ | 3.15 | 6.19 × 10−3 | 0.760 | 75.59 | 4.469 × 10−3 | 0.999 |
| AC-RH | DFC | 10.46 | 3.32 × 10−3 | 0.902 | 355.9 | 1.655 × 10−3 | 0.999 |
| AC-RH@Pectin | DFC | 35.58 | 6.11 × 10−3 | 0.956 | 127.6 | 4.901 × 10−4 | 0.999 |
| Samples | Adsorbate | qmaxexp (mg∙g−1) | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|---|---|
| qmax (mg∙g−1) | KL (L∙mg−1) | R2 | 1/n | KF (L1/n∙mg1−1/n∙g−1) | R2 | |||
| AC-RH | Pb2+ | 42.1 | 52.7 | 9.98 × 10−3 | 0.998 | 0.5481 | 1.83 | 0.963 |
| Pectin | Pb2+ | 190.7 | 245.7 | 2.898 × 10−2 | 0.992 | 0.6483 | 10.66 | 0.981 |
| AC-RH@Pectin | Pb2+ | 181.9 | 227.8 | 3.109 × 10−2 | 0.997 | 0.6424 | 10.35 | 0.966 |
| AC-RH | DFC | 514.5 | 537.6 | 1.491 × 10−2 | 0.999 | 0.3117 | 62.89 | 0.879 |
| AC-RH@Pectin | DFC | 118.3 | 130.9 | 4.177 × 10−3 | 0.997 | 0.2915 | 13.42 | 0.936 |
| Adsorbent | Adsorbate | SBET, m2/g | Adsorption Capacity, Langmuir qmax (mg/g) | Reference |
|---|---|---|---|---|
| AC from orange peels | DFC | 618 | 62.5 | [40] |
| AC from cocoa shell | DFC | 619 | 63.5 | [41] |
| AC from potato peel waste | DFC | 866 | 68.5 | [42] |
| AC Calgon Filtrasorb 400 | DFC | 997 | 280 | [43] |
| Synthetic AC “PC-1000” | DFC | 1236 | 392.0 | [44] |
| AC-RH (from rice husk) | DFC | 2938 | 537.6 | Present work |
| AC-RH@pectin composite | DFC | 256.8 | 130.0 | Present work |
| AC-RH (from rice husk) | Pb2+ | 2938 | 52.7 | Present work |
| Citrus GENU®Pectin LM | Pb2+ | 2.6 | 120.2 | [45] |
| DE SB pectin | Pb2+ | N/A | 129.9 | [33] |
| DE SB/A pectin | Pb2+ | 16.9 | 245.7 | Present work |
| DE citrus pectin | Pb2+ | N/A | 624.8 | [46] |
| Pectin/AC (2:3) | Pb2+ | 344.3 | 279.3 | [45] |
| AC-RH@pectin composite | Pb2+ | 256.8 | 227.8 | Present work |
| Model | Linear Equation | Parameters | Reference |
|---|---|---|---|
| Pseudo-first-order | qt (mg/g): adsorption capacity at a time point t qe (mg/g): adsorption capacity at equilibrium k1 (min−1): pseudo-first-order kinetic constant | [45] | |
| Pseudo-second-order | qt (mg/g): adsorption capacity at a time point t qe (mg/g): adsorption capacity at equilibrium k2 (min−1): pseudo-second-order kinetic constant | [51] | |
| Langmuir | qe (mg/g): adsorption capacity at the equilibrium Ce (mg/L): equilibrium concentration of adsorbate KL (L/mg): Langmuir constant qmax (mg/g): maximum adsorption capacity of the adsorbent | [46] | |
| Freundlich | KF: Freundlich constant nF: adsorption intensity | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jandosov, J.; Alavijeh, M.; Sultakhan, S.; Baimenov, A.; Bernardo, M.; Sakipova, Z.; Azat, S.; Lyubchyk, S.; Zhylybayeva, N.; Naurzbayeva, G.; et al. Activated Carbon/Pectin Composite Enterosorbent for Human Protection from Intoxication with Xenobiotics Pb(II) and Sodium Diclofenac. Molecules 2022, 27, 2296. https://doi.org/10.3390/molecules27072296
Jandosov J, Alavijeh M, Sultakhan S, Baimenov A, Bernardo M, Sakipova Z, Azat S, Lyubchyk S, Zhylybayeva N, Naurzbayeva G, et al. Activated Carbon/Pectin Composite Enterosorbent for Human Protection from Intoxication with Xenobiotics Pb(II) and Sodium Diclofenac. Molecules. 2022; 27(7):2296. https://doi.org/10.3390/molecules27072296
Chicago/Turabian StyleJandosov, Jakpar, Mo Alavijeh, Shynggyskhan Sultakhan, Alzhan Baimenov, Maria Bernardo, Zuriyadda Sakipova, Seytkhan Azat, Svitlana Lyubchyk, Nurzhamal Zhylybayeva, Gulmira Naurzbayeva, and et al. 2022. "Activated Carbon/Pectin Composite Enterosorbent for Human Protection from Intoxication with Xenobiotics Pb(II) and Sodium Diclofenac" Molecules 27, no. 7: 2296. https://doi.org/10.3390/molecules27072296
APA StyleJandosov, J., Alavijeh, M., Sultakhan, S., Baimenov, A., Bernardo, M., Sakipova, Z., Azat, S., Lyubchyk, S., Zhylybayeva, N., Naurzbayeva, G., Mansurov, Z., Mikhalovsky, S., & Berillo, D. (2022). Activated Carbon/Pectin Composite Enterosorbent for Human Protection from Intoxication with Xenobiotics Pb(II) and Sodium Diclofenac. Molecules, 27(7), 2296. https://doi.org/10.3390/molecules27072296