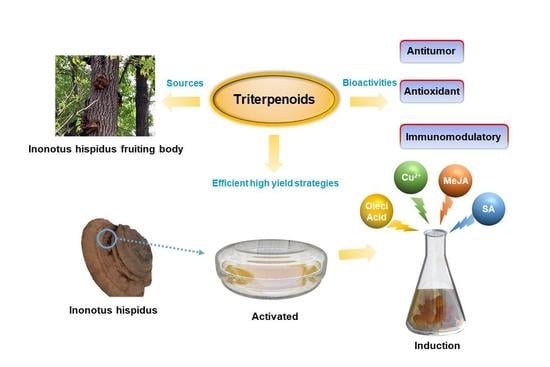
Effects of Compound Elicitors on the Biosynthesis of Triterpenoids and Activity of Defense Enzymes from Inonotus hispidus (Basidiomycetes)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Strain Cultivation
2.3. Assay of Mycelial Biomass
2.4. Extraction and Determination of Triterpenoids
2.5. Combination of Elicitors
2.5.1. Combination of SA and MeJA
2.5.2. Combination of MeJA and Oleic Acid
2.5.3. Combination of SA and Oleic Acid
2.5.4. Combination of SA and Cu2+
2.5.5. Combination of MeJA and Cu2+
2.5.6. Combination of Oleic Acid and Cu2+
2.5.7. Testing for Optimal Elicitor Compounds
2.6. Effect of Compound Elicitors on Defensive Enzymatic Activities
2.6.1. Determination of SOD Activity
2.6.2. Determination of CAT Activity
2.6.3. Determination of PAL Activity
2.7. Statistical Analysis
3. Results
3.1. Effects of Different Compound Elicitors on the Triterpenoid and Mycelial Content of I. hispidus
3.1.1. Effects of Induction by SA and MeJA on the Biosynthesis of Triterpenoids
3.1.2. Effects of Induction by MeJA and Oleic Acid on the Biosynthesis of Triterpenoids
3.1.3. Effects of Induction by SA and Oleic Acid on the Biosynthesis of Triterpenoids
3.1.4. Effects of Induction by SA and Cu2+ on the Biosynthesis of Triterpenoids
3.1.5. Effects of Induction by MeJA and Cu2+ on the Biosynthesis of Triterpenoids
3.1.6. Effects of Induction by Oleic Acid and Cu2+ on the Biosynthesis of Triterpenoids
3.1.7. Determining the Most Optimal Compound Elicitors
3.2. Effect of Oleic Acid and MeJA on Defensive Enzymatic Activities
3.2.1. Effect of Oleic Acid and MeJA on SOD Activity
3.2.2. Effect of Oleic Acid and MeJA on CAT Activity
3.2.3. Effect of Oleic Acid and MeJA on PAL Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, H.; He, W.; Li, D.; Bao, Y.; Riaz, A.; Xiao, Y.; Song, J.; Liu, C. Effect of methyl jasmonate on carotenoids biosynthesis in germinated maize kernels. Food Chem. 2020, 307, 125–525. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, L.; Chen, L.; Wu, X.; Lin, W.; Zheng, M.; Fu, J. Optimization on fermentation conditions for high production of Inonotus hispidus exopolysaccharides using Vernonia amygdalina dried leaf powder as additive and analyses on antioxidant activities of the polysaccharide product. Mygosystema 2019, 38, 403–413. [Google Scholar]
- Audrey, S.C.; Michele, F.; Unnikrishnan, K.; Jack, A.; Mathieu, P.; Peter, J.R. Methyl jasmonate treatment affects the regulation of the 2-C-methyl-D-erythritol 4-phosphate pathway and early steps of the triterpenoid biosynthesis in Chlamydomonas reinhardtii. Algal Res. 2019, 39, 101–462. [Google Scholar]
- Audrey, S.C.; Unnikrishnan, K.; Andrei, H.; Michele, F.; Ana Cristina, J.M.; Raffaela, M.A.; Peter, J.R.; Mathieu, P. Methyl Jasmonate and Methyl-β-Cyclodextrin Individually Boost Triterpenoid Biosynthesis in Chlamydomonas Reinhardtii UVM4. Pharmaceuticals 2021, 14, 20–125. [Google Scholar]
- Bernd, G.; Günther, S.; Guy De, B.; Eric, B.; Rafael, C.; Chapman, M.J.; John, D.; Olivier, S.D.; Ernst, R.R.; Karen, C.D.; et al. Plant sterols and cardiovascular disease: A systematic review and meta-analysis. Eur. Heart J. 2012, 33, 444–451. [Google Scholar]
- Renda, G.; Gökkaya, I.; Hretolu, D. Immunomodulatory properties of triterpenes. Phytochem. Rev. 2021, 4, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Rong, W.K.; Bing, X.; Zhi, J.W.; Jian, N.L.; Jun, R.Y.; Yu, Q.G.; Xia, Y.; Jin, M.G. Triterpenoids and meroterpenoids from the edible Ganoderma resinaceum and their potential anti-inflammatory, antioxidant and anti-apoptosis activities. Bioorganic Chem. 2022, 121, 105–689. [Google Scholar]
- Vergopoulou, S.; Galanopoulou, D.; Markaki, P. Methyl jasmonate stimulates aflatoxin B1 biosynthesis by Aspergillus parasiticus. J. Agric. Food Chem. 2001, 49, 3494–3498. [Google Scholar] [CrossRef]
- Lou, H.H.; Li, H.W.; Tian, Y.; Chen, Q.H. Stimulatory Effects of Oleic Acid and Fungal Elicitor on Betulinic Acid Production by Submerged Cultivation of Medicinal Mushroom Inonotus obliquus. J. Fungi 2021, 7, 266. [Google Scholar] [CrossRef]
- Zhong, Q.L.; Hu, H.L.; Fan, B.F.; Zhu, C.; Chen, Z.X. Biosynthesis and Roles of Salicylic Acid in Balancing Stress Response and Growth in Plants. Int. J. Mol. Sci. 2021, 22, 11672. [Google Scholar] [CrossRef]
- Kong, L.W.; Gao, Y.Q.; Xia, B.; Wang, J.Y.; Liu, X.N.; Tang, J.J.; Yin, X.; Gao, J.M. Ganoderterpene A, a New Triterpenoid from Ganoderma lucidum, Attenuates LPS-Induced Inflammation and Apoptosis via Suppressing MAPK and TLR-4/NF-κB Pathways in BV-2 Cells. J. Agric. Food Chem. 2021, 69, 12730–12740. [Google Scholar]
- Tessa, M.; Jacob, P.; Johan, M.T.; Alain, G. Bioengineering of plant (tri)terpenoids: From metabolic engineering of plants to synthetic biology in vivo and in vitro. New Phytol. 2013, 200, 27–43. [Google Scholar]
- Monika, C.; Israel, M.-L.; Nicolas, E.F.; Itzell, E.H.-S. Plant stress granules: Trends and Beyond. Front. Plant Sci. 2021, 12, 722643. [Google Scholar]
- Bhatt, M.; Pandey, S.S.; Tiwari, A.K.; Tiwari, B.S. Plastid-mediated singlet oxygen in regulated cell death. Plant Biol. 2021, 23, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.M.; Luo, B.B.; Yang, Y.; Mohammad Omar, F.; Zhang, J.L.; Li, X.H.; Hu, X.B. Addition of Vegetable Oil to Improve Triterpenoids Production in Liquid Fermentation of Medicinal Fungus Antrodia cinnamomea. J. Fungi 2021, 7, 926. [Google Scholar]
- Ahmed, E.; Szilárd, S.; Zoltán, S.; Attila, H.; Dorina, B.; Imre, J.H. Temporal Patterns and Inter-Correlations among Physical and Antioxidant Attributes and Enzyme Activities of Apricot Fruit Inoculated with Monilinia laxa under Salicylic Acid and Methyl Jasmonate Treatments under Shelf-Life Conditions. J. Fungi 2021, 7, 341. [Google Scholar]
- Xu, H.; Wang, Z. Optimization of the extraction technology of the triterpenoid from Inonotus hispidus by response surface. Sci. Technol. Food Ind. 2013, 34, 228–232. [Google Scholar]
- Xu, H.; Wang, Z. Optimizatiaon of submerged fermentation process of Inonotus hispidus for the yield of intracellular triterpenoid. Sci. Technol. Food Ind. 2012, 33, 206–214. [Google Scholar]
- Ueda, Y.; Uehara, N.; Sasaki, H.; Kobayashi, K.; Yamakawa, T. Impacts of acute ozone stress on superoxide dismutase (SOD) expression and reactive oxygen species (ROS) formation in rice leaves. Plant Physiol. Biochem. 2013, 70, 396–402. [Google Scholar] [CrossRef]
- Ying, W.; Robyn, B.; Alycia, N.; Siegfried, H. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar]
- Adrian, D.; Klaudia, G.-D.; Urszula, W.; Katarzyna, G.; Marian, W. The Associations between Leaf Morphology, Phenylalanine Ammonia Lyase Activity, Reactive Oxygen Species, and Fusarium Resistance in Selected Species of Wheat with Different Ploidy Levels. Plants 2019, 8, 360. [Google Scholar]
- Lander, B.; Boris, S.; Godelieve, G. Pathogens pulling the strings: Effectors manipulating salicylic acid and phenylpropanoid biosynthesis in plants. Mol. Plant Pathol. 2021, 22, 1436–1448. [Google Scholar]
- Chase, K.; Joe, C. Engineering triterpene metabolism in the oilseed of Arabidopsis thaliana. Plant Biotechnol. J. 2018, 17, 286–396. [Google Scholar]
- Qiu, Y.D.; Lukas, W.; Bader, O.A.; Jörg, K. Ca2+ signaling in plant responses to abiotic stresses. J. Integr. Plant Biol. 2022, 64, 287–300. [Google Scholar]
- Nguyen Hoang, L.; Nguyen Thanh, G. Effects of elicitors on the enhancement of asiaticoside biosynthesis in cell cultures of centella (Centella asiatica L. Urban). Chem. Pap. 2012, 66, 642–648. [Google Scholar]
- Lin, L.K.; Yuan, K.L.; Huang, Y.D.; Dong, H.Z.; Qiao, Q.H.; Xing, C.H.; Huang, X.S.; Zhang, S.L. A WRKY transcription factor PbWRKY40 from Pyrus betulaefolia functions positively in salt tolerance and modulating organic acid accumulation by regulating PbVHA-B1 expression. Environ. Exp. Bot. 2022, 196, 104–782. [Google Scholar] [CrossRef]
- Qi, H.C.; Jing, L.; Hai, F.Z.; Guo, Q.H.; Ming, L.F. The betulinic acid production from betulin through biotransformation by fungi. Enzym. Microb. Technol. 2009, 45, 175–180. [Google Scholar]
- Hyeonbae, L.; Jaehyun, P.; Han Min, W. Overexpression of the key enzymes in the methylerythritol-4-phosphate pathway in Corynebacterium glutamicum for improving farnesyl diphosphate-derived terpene production. J. Agric. Food Chem. 2020, 68, 10780–10796. [Google Scholar]
- You, H.; Sun, B.; Li, N.; Xu, J.W. Efficient expression of heterologous genes by the introduction of the endogenous glyceraldehyde-3-phosphate dehydrogenase gene intron 1 in Ganoderma lucidum. Microb. Cell Factories 2021, 20, 164–165. [Google Scholar] [CrossRef]
- Joshua, L.H.; Rachel, E.P.; Rachel, N.H.; Timothy, B.S.; Steven, J.S.; David, K.G. Exogenous polyunsaturated fatty acids (PUFAs) promote changes in growth, phospholipid composition, membrane permeability and virulence phenotypes in Escherichia coli. BMC Microbiol. 2020, 20, 1–21. [Google Scholar]
- Yenisleidy de Las Mercedes Zulueta, D.; Ambroggio, E.E.; Fanani, M.L. Miltefosine inhibits the membrane remodeling caused by phospholipase action by changing membrane physical properties. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183–407. [Google Scholar]
- Ham, S.Y.; Kim, H.S.; Jang, Y.; Ryoo, H.S.; Park, H.D. Synergistic control of membrane biofouling using linoleic acid and sodium hypochlorite. Chemosphere 2020, 268, 128802. [Google Scholar] [CrossRef]
- Ren, L.; Wang, M.R.; Wang, Q.C. ROS-induced oxidative stress in plant cryopreservation: Occurrence and alleviation. Planta 2021, 254, 1–124. [Google Scholar] [CrossRef]
- Monika, H.; Thomas, S. Stress priming, memory, and signalling in plants. Plant Cell Environ. 2019, 42, 753–761. [Google Scholar]
- Rui, J.; Yanping, W.; Ruijie, L.; Junbo, G.; Zhu, L.C. Physiological and Metabolic Changes of Purslane (Portulaca oleracea L.) in Response to Drought, Heat, and Combined Stresses. Front. Plant Sci. 2016, 6, 11–23. [Google Scholar]
- Borgstahl, G.; Oberley-Deegan, R. Superoxide Dismutases (SODs) and SOD Mimetics. Antioxidants 2018, 7, 156. [Google Scholar] [CrossRef] [Green Version]
- Huan, Z.; Wan, J.L.; Xin, D.Z.; Xu, L.; Dazuo, Y.; Hong, W.R.; Yi, B.Z. Cu/Zn superoxide dismutase (SOD) and catalase (CAT) response to crude oil exposure in the polychaete Perinereis aibuhitensis. Environ. Sci. Pollut. Res. 2016, 24, 616–627. [Google Scholar]
- Pavel, I.K.; Frank Van, B. Improving oxidative stress resilience in plants. Plant J. 2021, 109, 359–372. [Google Scholar]








| Day | Activity of SOD(U/mgprot) under the Treatment with Different Elicitors | |||
|---|---|---|---|---|
| Control | 2% of Oleic Acid | 100 μmol/L MeJA | 2% of Oleic Acid+ 100 μmol/L MeJA | |
| 0 | 48.34 ± 2.20 aC | 54.56 ± 2.45 aD | 48.34 ± 2.20 aC | 54.56 ± 2.45 aD |
| 2 | 55.27 ± 3.03 bC | 78.70 ± 3.54 bD | 55.27 ± 3.03 bC | 78.70 ± 3.54 bD |
| 3 | 52.74 ± 2.24 bC | 71.86 ± 2.10 bD | 52.74 ± 2.24 bC | 71.86 ± 2.10 bD |
| 5 | 47.47 ± 2.78 aC | 60.84 ± 2.13 cD | 47.47 ± 2.78 aC | 60.84 ± 2.13 aD |
| 7 | 45.60 ± 2.13 aC | 52.25 ± 2.03 aC | 59.70 ± 3.10 cD | 68.69 ± 3.01 cF |
| 8 | 42.40 ± 1.45 cC | 47.40 ± 2.01 aC | 56.39 ± 2.89 bD | 63.30 ± 2.97 aF |
| 10 | 42.10 ± 1.98 cC | 45.20 ± 2.41 aC | 54.60 ± 1.56 bD | 60.30 ± 2.45 aF |
| Day | Activity of CAT (U/mgprot) under Treatment with Different Elicitors | |||
|---|---|---|---|---|
| Control | 2% of Oleic Acid | 100 μmol/L MeJA | 2% of Oleic Acid+ 100 μmol/L MeJA | |
| 0 | 14.57 ± 1.53 aC | 16.57 ± 1.41 aD | 14.57 ± 1.53 aC | 16.57 ± 1.41 aC |
| 2 | 19.69 ± 2.43 aC | 28.33 ± 2.32 bD | 19.69 ± 2.43 aC | 28.33 ± 2.32 bD |
| 3 | 25.20 ± 1.50 bC | 38.50 ± 3.45 cD | 25.20 ± 1.50 bC | 38.50 ± 3.45 cD |
| 5 | 23.20 ± 2.61 bC | 29.82 ± 2.67 bD | 23.20 ± 2.61 bC | 29.82 ± 2.67 bD |
| 7 | 20.32 ± 1.57 bC | 26.80 ± 2.50 bD | 28.40 ± 2.56 bD | 34.90 ± 1.70 bF |
| 8 | 18.83 ± 1.49 aC | 23.30 ± 1.39 aC | 32.80 ± 3.46 cD | 37.08 ± 1.97 cF |
| 10 | 18.16 ± 1.47 aC | 22.17 ± 1.40 aD | 30.32 ± 2.57 cF | 32.32 ± 1.29 bF |
| Day | Activity of PAL (U/mgprot) under Treatment with Different Elicitors | |||
|---|---|---|---|---|
| Control | 2% of Oleic Acid | 100 μmol/L MeJA | 2% of Oleic Acid+ 100 μmol/L MeJA | |
| 0 | 25.56 ± 3.20 aD | 29.56 ± 3.07 bD | 25.56 ± 3.20 aC | 29.56 ± 3.07 aD |
| 2 | 30.81 ± 3.65 bC | 35.40 ± 4.05 cD | 30.81 ± 3.65 aC | 35.40 ± 4.05 bD |
| 3 | 29.07 ± 2.96 bC | 40.52 ± 4.42 cD | 29.07 ± 2.96 aC | 40.52 ± 4.42 cD |
| 5 | 27.78 ± 2.69 bC | 32.64 ± 3.32 cD | 27.78 ± 2.69 aC | 32.64 ± 3.32 bD |
| 7 | 25.40 ± 2.21 aC | 28.47 ± 3.90 bD | 34.62 ± 3.12 cF | 38.88 ± 3.77 cF |
| 8 | 24.20 ± 4.01 aC | 26.77 ± 4.07 aC | 32.41 ± 3.20 bF | 33.45 ± 3.21 bF |
| 10 | 23.09 ± 3.10 aC | 25.07 ± 3.65 aC | 29.20 ± 2.01 aD | 31.70 ± 2.20 aD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Lin, X.; Liu, S.; Wang, Z.; Liu, D.; Huo, Y.; Li, D. Effects of Compound Elicitors on the Biosynthesis of Triterpenoids and Activity of Defense Enzymes from Inonotus hispidus (Basidiomycetes). Molecules 2022, 27, 2618. https://doi.org/10.3390/molecules27092618
Zhou J, Lin X, Liu S, Wang Z, Liu D, Huo Y, Li D. Effects of Compound Elicitors on the Biosynthesis of Triterpenoids and Activity of Defense Enzymes from Inonotus hispidus (Basidiomycetes). Molecules. 2022; 27(9):2618. https://doi.org/10.3390/molecules27092618
Chicago/Turabian StyleZhou, Jiao, Xinyue Lin, Shuangshuang Liu, Zhanbin Wang, Dongchao Liu, Yonghong Huo, and Dehai Li. 2022. "Effects of Compound Elicitors on the Biosynthesis of Triterpenoids and Activity of Defense Enzymes from Inonotus hispidus (Basidiomycetes)" Molecules 27, no. 9: 2618. https://doi.org/10.3390/molecules27092618
APA StyleZhou, J., Lin, X., Liu, S., Wang, Z., Liu, D., Huo, Y., & Li, D. (2022). Effects of Compound Elicitors on the Biosynthesis of Triterpenoids and Activity of Defense Enzymes from Inonotus hispidus (Basidiomycetes). Molecules, 27(9), 2618. https://doi.org/10.3390/molecules27092618