Chemical Characteristics of Ethanol and Water Extracts of Black Alder (Alnus glutinosa L.) Acorns and Their Antibacterial, Anti-Fungal and Antitumor Properties
Abstract
:1. Introduction
2. Results and Discussion
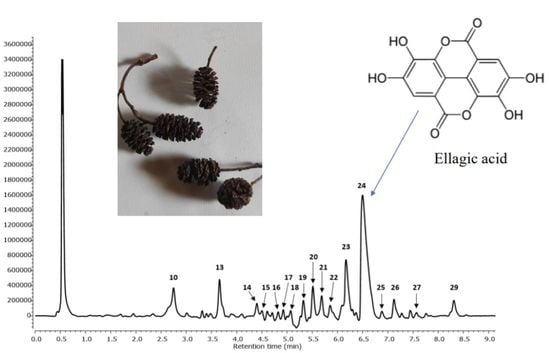
2.1. Identification of Phenolic Compounds in Ethanol and Aqueous Extracts of A. Glutinosa Acorns
2.1.1. Ellagitannins
2.1.2. Ellagic Acid Derivatives
2.1.3. Phenolic Acids
2.1.4. Flavonols
2.2. Quantitative Analysis of Polyphenols
2.3. Effect of the Tested Extracts on Growth of Tumor Cell Line
2.4. Antimicrobial Activity
3. Materials and Methods
3.1. Reagents and Standards
3.2. Plant Material
3.3. Extraction Procedure
3.4. Determination of Phenolic Compounds
Identification and Quantification of Phenolic Compounds by the UPLC-PDA–MS Method
3.5. Determination of Biological Properties
3.5.1. Cell Culture
3.5.2. Bacterial Strains
3.5.3. Growth Inhibition of Tumor Cell Lines
3.5.4. Colorimetric MTT Assay for Cell Growth and Viability
3.5.5. Determination of Antimicrobial Activity
3.5.6. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ren, X.; He, T.; Chang, Y.; Zhao, Y.; Chen, X.; Bai, S.; Wang, L.; Shen, M.; She, G. The Genus Alnus, A Comprehensive Outline of Its Chemical Constituents and Biological Activities. Molecules 2017, 22, 1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahija, S.; Čakar, J.; Vidic, D.; Maksimović, M.; Parić, A. Total phenolic and flavo-noid contents, antioxidant and antimicrobial activities of Alnus glutinosa (L.) Gaertn., Alnus incana (L.) Moench and Alnus viridis (Chaix) DC. extracts. Nat. Prod. Res. 2014, 28, 2317–2320. [Google Scholar] [CrossRef] [PubMed]
- Abedini, A.; Chollot, S.; Angelis, A.; Borie, N.; Nuzillard, J.M.; Skaltsounis, A.L.; Reynaud, R.; Gangloff, S.C.; Renault, J.H.; Hubert, J. Bioactivity-guided identification of antimicrobial metabolites in Alnus glutinosa bark and optimization of oregonin purification by Centrifugal Partition Chromatography. J. Chromatogr. B 2016, 1, 1029–1030. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Webster, D.; Johnson, J.A.; Gray, C.A. Anti-mycobacterial triterpenes from the Canadian medicinal plant Alnus incana. J. Ethnopharmacol. 2015, 165, 148–151. [Google Scholar] [CrossRef]
- Adebooye, O.C.; Alashi, A.M.; Aluko, R.E. A brief review on emerging trends in global polyphenol research. J. Food Biochem. 2018, 42, e12519. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Đorđević, V.B.; Zdunić, G.M.; Pljevljakušić, D.S.; Šavikin, K.P.; Gođevac, D.M.; Bugarski, B.M. Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration-, heat and ultrasound-assisted techniques. Sep. Purif. Technol. 2017, 179, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of extraction time, temperatureand solvent on concentration and antioxidant activity of grape marcphenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Hammoudi, R.; Khenfer, S.; Medjouel, M.; Tlili, M.L.; Mahammed, M.H. Optimization of extraction conditions for phenolic compound from Salvia chudaei. Leban. Sci. J. 2017, 18, 234–243. [Google Scholar]
- De la Cruz, J.F.; Kim, Y.S.; Lumbera, W.M.; Hwang, S.G. Viscum Album Var Hot Water Extract Mediates Anti-cancer Effects through G1 Phase Cell Cycle Arrest in SK-Hep1 HumanHepatocarcinoma cells. Asian Pac. J. Cancer Prev. 2015, 16, 6417–6421. [Google Scholar] [CrossRef] [Green Version]
- Vijaybabu, K.; Punnagai, K. In-vitro anti-proliferative effects of ethanolic extract of Vanilia planifoia leaf extract against A431 human epideromoid carcinoma cells. Biomed. Pharm. J. 2019, 12, 1141–1146. [Google Scholar]
- Lim, E.G.; Kim, G.T.; Lee, S.H.; Kim, S.Y.; Kim, Y.M. Apoptotic effects of extract from Cnidium monnieri (L.) Cusson by adenosine monosphosphate-activatedprotein kinase-independent pathway in HCT116 colon cancer cells. Mol. Med. Rep. 2016, 13, 4681–4688. [Google Scholar] [CrossRef] [PubMed]
- Vergara, D.; Simeone, P.; Bettini, S.; Tinelli, A.; Valli, L.; Storelli, C.; Leo, S.; Santino, A.; Maffia, M. Antitumor activity of the dietary diterpene carnosol against a panel of human cancer cell lines. Food Funct. 2014, 5, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.G.; Kim, G.T.; Kim, B.M.; Kim, E.J.; Kim, S.Y.; Kim, Y.M. Ethanol extract from Cnidium monnieri (L.) Cusson induces cell cycle arrest and apoptosis via regulation of the p53-independent pathway in HepG2 and Hep3B hepatocellular carcinoma cells. Mol. Med. Rep. 2018, 17, 2572–2780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, S.S.; Kim, M.S.; Oh, W.S.; Lee, D.I. Enhancement of NK cytotoxicity, anti-metastasis and elongation effect of survival time in B16-F10 melanoma cells by oregonin. Arch. Pharmacal Res. 2002, 25, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Nanchal, R.; Subramanian, R.; Ison, M.G.; Heldman, M. Bacterial Infections. Hepatic Crit. Care 2017, 7, 191–200. [Google Scholar] [CrossRef]
- Aelenei, A.; Miron, A.; Trifan, A.; Bujor, A.; Gille, A.; Aprotosoaie, A.C. Essential Oils and Their Components as Modulators of Antibiotic Activity against Gram-Negative Bacteria. Medicines 2016, 3, 19. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, R.; Rolta, R.; Dev, K.; Sourirajan, A. Synergistic potential for essential oils with antibiotics to combat fungal pathogenic: Present status and future perspectives. Phytother. Res. 2021, 35, 6089–6100. [Google Scholar] [CrossRef]
- Zhang, B.; Cai, J.; Duan, C.-Q.; Reeves, M.J.; He, F. A Review of Polyphenolics in Oak Woods. Int. J. Mol. Sci. 2015, 16, 6978–7014. [Google Scholar] [CrossRef]
- Mena, P.; Calani, L.; Dall’Asta, C.; Galaverna, G.; García-Viguera, C.; Bruni, R.; Crozier, A.; Del Rio, D. Rapid and Comprehensive Evaluation of (Poly)phenolic Compounds in Pomegranate (Punica granatum L.) Juice by UHPLC-MSn. Molecules 2012, 17, 14821–14840. [Google Scholar] [CrossRef] [Green Version]
- Sobeh, M.; ElHawary, E.; Peixoto, H.; Labib, R.M.; Handoussa, H.; Swilam, N.; El-Khatib, A.A.; Sharapov, F.; Mohamed, T.; Krstin, S.; et al. Identification of phenolic secondary metabolites from Schotia brachypetala Sond. (Fabaceae) and demonstration of their antioxidant activities in Caenorhabditis elegans. Peer J. 2016, 4, e2404. [Google Scholar] [CrossRef] [Green Version]
- Molina-García, L.; Martínez-Expósito, R.L.; Fernández-de Córdova, M.L.; Llorent-Martínez, E.J. Determination of the Phenolic Profile and Antioxidant Activity of Leaves and Fruits of Spanish Quercus coccifera. J. Chem. 2018, 2018. [Google Scholar] [CrossRef]
- Wyrepkowski, C.C.; Gomes da Costa, D.L.M.; Sinhorin, A.P.; Vilegas, W.; De Grandis, R.A.; Resende, F.A.; Varanda, E.A.; Campaner dos Santos, L. Characterization and Quantification of the Compounds of the Ethanolic Extract from Caesalpinia ferrea Stem Bark and Evaluation of Their Mutagenic Activity. Molecules 2014, 19, 16039–16057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carocho, M.; Barros, L.; Bento, A.; Santos-Buelga, C.; Morales, P.; Ferreira, I.C.F.R. Castanea sativa Mill. Flowers amongst the Most Powerful Antioxidant Matrices: A Phytochemical Approach in Decoctions and Infusions. BioMed Res. Int. 2014, 2014, 232956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Bubba, M.; Checchini, L.; Chiuminatto, U.; Doumett, S.; Fibbi, D.; Giordan, E. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of polyphenolic composition of four cultivars of Fragaria vesca L. berries and their comparative evaluation. J. Mass Spectrom. 2012, 47, 1207–1220. [Google Scholar] [CrossRef]
- Fernández-Poyatos, M.P.; Ruiz-Medina, A.; Zengin, G.; Llorent-Martínez, E.J. Phenolic Characterization, Antioxidant Activity, and Enzyme Inhibitory Properties of Berberis thunbergii DC. Leaves: A Valuable Source of Phenolic Acids. Molecules 2019, 24, 4171. [Google Scholar] [CrossRef] [Green Version]
- Złotek, U.; Mikulska, S.; Nagajek, M.; Świeca, M. The effect of different solvents and number of extraction steps on the polyphenol content and antioxidant capacity of basil leaves (Ocimum basilicum L.) extracts. Saudi J. Biol. Sci. 2016, 23, 628–633. [Google Scholar] [CrossRef]
- Koffi, E.; Sea, T.; Dodehe, Y.; Soro, S. Effect of solvent type on extraction of polyphenols from twenty-three Ivorian plants. J. Anim. Plant Sci. 2010, 5, 550–558. [Google Scholar]
- Onyebuchi, C.; Kavaz, D. Effect of extraction temperature and solvent type on the bioactive potential of Ocimum gratissimum L. extracts. Sci. Rep. 2020, 10, 149–155. [Google Scholar] [CrossRef]
- Zeroual, A.; Sakar, E.H.; Eloutassi, N.; Mahjoubi, F.; Chaouch, M.; Chaqroune, A. Wild Chamomile [Cladanthus Mixtus (L.) Chevall.] Collected from Central-Northern Morocco: Phytochemical Profiling, Antioxidant, and Antimicrobial Activities. Biointerface Res. Appl. Chem. 2021, 11, 11440–11457. [Google Scholar] [CrossRef]
- Zeroual, A.; Sakar, E.H.; Mahjoubi, F.; Chaouch, M.; Chaqroune, A.; Taleb, M. Effects of Extraction Technique and Solvent on Phytochemicals, Antioxidant, and Antimicrobial Activities of Cultivated and Wild Rosemary (Rosmarinus officinalis L.) from Taounate Region (Northern Morocco). Biointerface Res. Appl. Chem. 2022, 12, 8441–8452. [Google Scholar] [CrossRef]
- Kobus, Z.; Wilczyński, K.; Nadulski, R.; Rydzak, L.; Guz, T. Effect of solvent polarity on the efficiency of ultrasound-assisted extraction of polyphenols from apple pomace. In Proceedings of the IX International Scientific Symposium “Farm Machinery and Processes Management in Sustainable Agriculture”, Lublin, Poland, 22–24 November 2017. [Google Scholar] [CrossRef]
- Azrie, A.M.; Chuah, A.L.; Pin, K.Y.; Tan, H.P. Effect of solvents on the extraction of Kacip Fatimah (Labisia pumila) leaves. J. Chem. Pharm. Res. 2014, 6, 172–176. [Google Scholar]
- Wijngaard, H.H.; Brunton, N. The optimisation of solid–liquid extraction of antioxidants from apple pomace by response surface methodology. J. Food Eng. 2010, 96, 134–140. [Google Scholar] [CrossRef]
- Pin, K.Y.; Chuah, T.G.; Abdullah Rashih, A.; Law, C.L.; Rasadah, M.A. Drying of Betel Leaves (Piper betle L.): Quality and Drying Kinetics. Dry. Technol. 2009, 27, 149–155. [Google Scholar] [CrossRef]
- Chun, S.S.; Vattem, D.A.; Lin, Y.T.; Shetty, K. Phenolic antioxidants from clonal oregano (Origanum vulgare) with antimicrobial activity against Helicobacter pylori. Proc. Biochem. 2005, 40, 809–816. [Google Scholar] [CrossRef]
- Rotelli, A.E.; Guardia, T.; Juarez, A.O.; De la Rocha, N.E.; Pelzer, L.E. Comparative study of flavonoids in experimental models of inflammation. Pharmacol. Res. 2003, 48, 601–606. [Google Scholar] [CrossRef]
- Wang, L.; Tu, Y.C.H.; Lian, T.W.; Hung, J.T.; Yen, J.H.; Wu, M.J. Distinctive antioxidant and antiinflammatory effects of flavonols. J. Agric. Food Chem. 2006, 54, 9798–9804. [Google Scholar] [CrossRef]
- Geng, W.; Narron, R.; Jiang, X.; Pawlak, J.J.; Chang, H.M.; Park, S.; Jameel, H.; Venditti, R.A. The Influence of Lignin Content and Structure on Hemicellulose Alkaline Extraction for Non-wood and Hardwood Lignocellulosic Biomass. Cellulose 2016, 26, 3219–3230. [Google Scholar] [CrossRef]
- Zheng, L.; Yu, P.; Zhang, Y.; Wang, P.; Yan, W.; Guo, B.; Huang, C.; Jiang, Q. Evaluating the Bio-Application of Biomacromolecule of Lignin-Carbohydrate Complexes (LCC) from Wheat Straw in Bone Metabolism via ROS Scavenging. Int. J. Biol. Macromol. 2021, 176, 13–25. [Google Scholar] [CrossRef]
- Mohansrinivasan, V.; Devi, C.; Deori, M.; Biswas, A.; Naine, S. Exploring the anticancer activity of grape seed extract on skin cancer cell Lines A431. Braz. Arch. Biol. Technol. 2015, 58, 540–546. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.; Zhou, J.; Jie, C.; Xing, D.; Zhang, Y. Anticancer activity and mechanism of Scutellaria barbata extract on human lung cancer cell line A549. Life Sci. 2004, 75, 2233–2244. [Google Scholar] [CrossRef]
- Sharma, P.; Montes de Oca, M.K.; Alkeswani, A.R.; McClees, S.F.; Das, T.; Elmets, C.A.; Afaq, F. Tea Polyphenols for the Prevention of UVB-Induced Skin Cancer. Photodermatol. Photoimmunol. Photomed. 2017, 34, 50–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sajadimajd, S.; Bahramsoltani, R.; Iranpanah, A.; Kumar Patra, J.; Das, G.; Gouda, S.; Rahimi, R.; Rezaeiamiri, E.; Cao, H.; Giampieri, F.; et al. Advances on Natural Polyphenols as Anticancer Agents for Skin Cancer. Pharmacol. Res. 2020, 151, 104584. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Bennett, L.L.; Zhou, S. Multifaceted Ability of Naturally Occurring Polyphenols against Metastatic Cancer. Clin. Exp. Pharmacol. Physiol. 2016, 43, 394–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, J.; Li, S.; Wang, C.; Cao, N.; Qu, H.; Cheng, C.; Wang, Z.; Wang, L.; Zhou, L. Potential Applications of Polyphenols on Main ncRNAs Regulations as Novel Therapeutic Strategy for Cancer. Biomed. Pharmacother. 2019, 113, 108703. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Leonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019, 10, 892. [Google Scholar] [CrossRef] [PubMed]
- Taguri, T.; Tanaka, T.; Kouno, I. Antibacterial spectrum of plant polyphenols and extracts depending upon hydroxyphenyl structure. Biol. Pharm. Bull. 2006, 29, 2226–2235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altinyay, C.; Eryilmaz, M.; Yazgan, A.N.; Sever Yilmaz, B.; Altun, M.L. Antimicrobial activity of some Alnus species. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4671–4674. [Google Scholar]
- An, B.-J.; Kwak, J.-H.; Son, J.-H.; Park, J.-M.; Lee, J.-Y.; Jo, C.; Byun, M.-W. Biological and Anti-microbial Activity of Irradiated green tea Polyphenols. Food Chem. 2004, 88, 549–555. [Google Scholar] [CrossRef]
- Gradisar, H.; Pristovsek, P.; Plaper, A.; Jerala, R. Green tea Catechins Inhibit Bacterial DNA Gyrase by Interaction with its ATP Binding Site. J. Med. Chem. 2007, 50, 264–271. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Ge, S.; Lin, S. Review of Distribution, Extraction Methods, and Health Benefits of Bound Phenolics in Food Plants. J. Agric. Food Chem. 2020, 68, 3330–3343. [Google Scholar] [CrossRef]
- Yun, J.; Wei, L.; Li, W.; Gong, D.; Qin, H.; Feng, X.; Li, G.; Li, Z.; Wang, P.; Yin, B. Isolating High Antimicrobial Ability Lignin from Bamboo Kraft Lignin by Organosolv Fractionation. Front. Bioeng. Biotechnol. 2021, 9, 683796. [Google Scholar] [CrossRef] [PubMed]
- Ramadas, D.; Kowti, R.; Ramesh, B.; Nune, S.K.; Joshi, V.; District, M. Antibacterial Effect of Polyphenols Enriched Drumstick Plant Leaves (Moringa Oleifera) Extract: A Research Study. World J. Adv. Res. Rev. 2020, 5, 1281–1283. [Google Scholar]
- Kolniak-Ostek, J.; Oszmiański, J. Characterization of phenolic compounds in different anatomical pear (Pyrus communis L.) parts by ultra-performance liquid chromatography photodiode detector-quadrupole/time of flight-mass spectrometry (UPLC-PDA-Q/TOF-MS). Int. J. Mass Spectrom. 2015, 392, 154–163. [Google Scholar] [CrossRef]
- Hansen, M.B.; Nielsen, S.E.; Berg, K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 1989, 119, 203–210. [Google Scholar] [CrossRef]

| Peak | Rt | λ (nm) | [M − H]− (m/z) b | MS/MS (m/z) b> | Tentative Identification | Water Extract | Ethanol Extract |
|---|---|---|---|---|---|---|---|
| 1. | 0.82 | 220 | 481.0628 | 300.9964/275.0187/257.0077/229.0715 | HHDP-hexoside(1-galloyl-2,3-HHDPl-α-glucose) | √ | |
| 2. | 0.92 | 324 | 355.0305 | 193.0130 | Ferulic acid hexoside | √ | |
| 3. | 1.07 | 222 | 481.0611 | 300.9986 | HHDP-hexoside | √ | |
| 4. | 1.16 | 273 | 649.0592 | 605.0702/479.0501/300.9987 | Trisgalloyl hexoside | √ | |
| 5. | 1.29 | 230 | 963.1341 | 933.0640/300.9985 | Methoxylated castalagin/vescalagin | √ | |
| 6. | 1.64 | 235/320 | 785.0615 | 633.0641/300.9984 | HHDP-digalloyl-hexoside | √ | |
| 7. | 1.71 | 274 | 783.0668 | 481.0624/300.9978 | Bis-HHDP-hexoside (pedunculagin isomer) | √ | |
| 8. | 1.95 | 280 | 483.0761 | 271.0187/193.0340/169.0134/125.0235 | Digalloyl-glucose | √ | |
| 9. | 2.34 | 274 | 783.0645 | 633.0770/481.0583/300.9964 | Bis-HHDP-hexoside (pedunculagin isomer) | √ | |
| 10. | 2.75 | 279 | 783.0702 | 481.0604; 300.9982 | Bis-HHDP-hexoside (pedunculagin isomer) | √ | √ |
| 11. | 3.30 | 235 | 633.0581 | 481.9926/300.9982 | HHDP-galloyl-hexoside | √ | |
| 12. | 3.65 | 326 | 367.0090 | 191.0128 | Methyl-caffeoyl-quinate | √ | |
| 13. | 3.66 | 274 | 783.0645 | 481.0613; 300.9984 | Bis-HHDP-hexoside (pedunculagin isomer) | √ | |
| 14. | 4.39 | 280 | 935.0596 | 783.0650; 633.0740 | Galloyl-bis-HHDP-hexoside | √ | √ |
| 15. | 4.5 | 235/320 | 785.0615 | 633.0641; 300.9984 | HHDP-digalloyl-hexoside | √ | √ |
| 16. | 4.82 | 325 | 633.0550 | 481.0470; 300.9982 | HHDP-galloyl-hexoside | √ | √ |
| 17. | 4.93 | 230 | 933.0406 | 915.0529; 633.0581; 450.9908; 301.0070 | Castalagin/vescalagin | √ | |
| 18. | 5.07 | 224 | 936.0582 | 300.9964 | Galloyl-bis-HHDP-hexoside | √ | |
| 19. | 5.32 | 279 | 783.0427 | 481.0117; 300.9964 | Bis-HHDP-hexoside (pedunculagin isomer) | √ | √ |
| 20. | 5.51 | 235 | 933.0645 | 450.9958; 301.0000 | Castalagin/vescalagin | √ | |
| 21. | 5.68 | 246 | 1869.1495 | 934.0719; 633.0584; 468.9935; 300.9985 | Dimer of galloyl-bis-HHDP-glucose (sanguiin H-6) | √ | √ |
| 22. | 5.85 | 230 | 933.0707 | 633.0404; 300.9991 | Castalagin/vescalagin | √ | |
| 23. | 6.17 | 252/364 | 433.0420 | 301.0354 | Ellagic acid pentoside | √ | √ |
| 24. | 6.5 | 255/365 | 300.9999 | 285.0425; 257.0208; 229.0137 | Ellagic acid | √ | √ |
| 25. | 6.87 | 225 | 935.0639 | 783.0604; 300.9987 | Galloyl-bis-HHDP-hexoside | √ | |
| 26. | 7.12 | 224 | 935.0799 | 783.0650; 300.9981 | Galloyl-bis-HHDP-hexoside | √ | |
| 27. | 7.56 | 350 | 447.0560 | 315.0143 | Isorhamnetin pentoside | √ | √ |
| 28. | 7.75 | 254/362 | 461.0121 | 300.9984 | Ellagic acid hexoside | √ | |
| 29. | 8.31 | 340 | 315.0133 | Isorhamnetin | √ | √ |
| Peak | Compound | Water | Ethanol |
|---|---|---|---|
| Ellagitannins | |||
| 1. | HHDP-hexoside (1-galloyl-2,3,hexahydroxydiphenoyl-α-glucose) | 43.43 ± 0.5 k | 0.00 ± 0.0 l |
| 3. | HHDP-hexoside | 143.35 ± 4.5 d | 0.00 ± 0.0 l |
| 4. | Trisgalloyl hexoside | 68.04 ± 1.2 i | 0.00 ± 0.0 l |
| 5. | Methoxylated castalagin/vescalagin | 97.50 ± 2.1 g | 0.00 ± 0.0 l |
| 6. | HHDP-digalloyl-hexoside | 173.31 ± 3.7 c | 0.00 ± 0.0 l |
| 7. | Bis-HHDP-hexoside (pedunculagin isomer) | 242.52 ± 5.5 a | 0.00 ± 0.0 l |
| 8. | Digalloyl-glucose | 82.31 ± 1.1 h | 0.00 ± 0.0 l |
| 9. | Bis-HHDP-hexoside (pedunculagin isomer) | 103.60 ± 2.3 f | 0.00 ± 0.0 l |
| 10. | Bis-HHDP-hexoside (pedunculagin isomer) | 225.91 ± 3.4 a | 199.84 ± 2.4 b |
| 11. | HHDP-galloyl-hexoside | 72.26 ± 1.0 i | 0.00 ± 0.0 l |
| 13. | Bis-HHDP-hexoside (pedunculagin isomer) | 0.00 ± 0.0 l | 173.24 ± 1.9 c |
| 14. | Galloyl-bis-HHDP-hexoside | 49.69 ± 1.2 k | 232.35 ± 2.2 a |
| 15. | HHDP-digalloyl-hexoside | 59.48 ± 1.1 j | 96.43 ± 0.8 g |
| 16. | HHDP-galloyl-hexoside | 63.44 ± 1.6 j | 105.95 ± 1.0 f |
| 17. | Castalagin/vescalagin | 0.00 ± 0.0 l | 102.70 ± 1.0 f |
| 18. | Galloyl-bis-HHDP-hexoside | 0.00 ± 0.0 l | 210.04 ± 1.6 b |
| 19. | Bis-HHDP-hexoside (pedunculagin isomer) | 108.28 ± 2.3 f | 208.00 ± 1.3 b |
| 20. | Castalagin/vescalagin | 0.00 ± 0.0 l | 215.44 ± 1.5 b |
| 21. | Dimer of galloyl-bis-HHDP-glucose (sanguiin H-6) | 59.99 ± 0.9 j | 206.01 ± 1.2 b |
| 22. | Castalagin/vescalagin | 0.00 ± 0.0 l | 183.46 ± 1.0 c |
| 25. | Galloyl-bis-HHDP-hexoside | 0.00 ± 0.0 l | 130.60 ± 0.9 e |
| 26. | Galloyl-bis-HHDP-hexoside | 0.00 ± 0.0 l | 107.83 ± 0.8 f |
| Sum | 1593.13 | 2171.90 | |
| Ellagic acid derivatives | |||
| 23. | Ellagic acid pentoside | 247.78 ± 10.2 d | 409.99 ± 15.6 c |
| 24. | Ellagic acid | 675.37 ± 19.7 a | 582.56 ± 13.3 b |
| 28. | Ellagic acid hexoside | 29.13 ± 0.2 e | 0.00 ± 0.0 f |
| Sum | 952.28 | 992.54 | |
| Phenolic acids | |||
| 2. | Ferulic acid hexoside | 68.48 ± 0.3 b | 0.00 ± 0.0 c |
| 12. | Methyl-caffeoyl-quinate | 195.09 ± 0.9 a | 0.00 ± 0.0 c |
| Sum | 263.57 | 0.00 | |
| Flavonols | |||
| 27. | Isorhamnetin pentoside | 19.80 ± 0.8 b | 53.25 ± 1.3 a |
| 29. | Isorhamnetin | 21.16 ± 0.7 b | 59.03 ± 1.5 a |
| Sum | 40.96 | 112.28 | |
| Sum of phenolic compounds | 2849.93 | 3276.72 |
| Dilution of Extract Stock Solution | The Percentage of Viability | |||
|---|---|---|---|---|
| A431 Cells | A549 Cells | |||
| Ethanol Extract | Water Extract | Ethanol Extract | Water Extract | |
| 1:400 | 51 ± 2 f | 37 ± 2 f | 77 ± 5 f | 58 ± 1 f |
| 1:800 | 61 ± 2 f | 40 ± 1 f | 77 ± 3 f | 62 ± 1 f |
| 1:1600 | 68 ± 6 f | 38 ± 1 f | 80 ± 1 f | 67 ± 1 f |
| 1:3200 | 77 ± 2 f | 55 ± 3 f | 95 ± 1 | 71 ± 2 f |
| Bacterial or Fungal Growth Inhibition Zone (mm) | ||||||
|---|---|---|---|---|---|---|
| Strains | Water Extract Dilution | Ethanol Extract Dilution | ||||
| 1:1 | 1:5 | 1:10 | 1:20 | 1:40 | 1:80 | |
| Escherichia coli PCM 2057 | 6 mm (clear zone) | 6 mm | 3 mm | 6 mm (clear zone) | 0 | 0 |
| (21 mm opaque) | ||||||
| Pseudomonas aeruginosa PCM 2058 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pseudomonas aeruginosa PCM 499 | 0 | 0 | 0 | 0 | 0 | 0 |
| Proteus mirabilis PCM 2958 | 0 | 0 | 0 | 0 | 0 | 0 |
| Citrobacter freundii PCM 2959 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staphylococcus aureus PCM 2054 | 12 mm clear zone | 11 mm (opaque) | 4 mm (opaque) | 5 mm (clear zone) | 5 mm (opaque) | 0 |
| Streptococcus mutans ATCC 25175 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bacillus subtilis PCM 2021 | 6 mm clear zone | 11 mm (opaque) | 0 | 5 mm (clear zone) | 4 mm (clear zone) | 0 |
| (21 mm opaque) | ||||||
| Candida albicans ATCC 90028 | 6 mm (opaque) | 0 | 0 | 4 mm opaque | 0 | 0 |
| Candida glabrata PCM 2703 | 12 mm (opaque) | 11 mm (opaque) | 4 mm (opaque) | 6 mm opaque | 5 mm (opaque) | 0 |
| Ethanol dilution equivalent | 0 | 0 | 0 | |||
| Bacteria | CFU/mL/Time | ||
|---|---|---|---|
| 0 h | 24 h | ||
| Control | Extract Alder Acorns | ||
| Staphylococcus aureus PCM 2054 | 1.0 × 107 | 7.5 × 107 | 7.0 × 105 * |
| Escherichia coli PCM 2057 | 2.0 × 108 | 6.0 × 108 | 4.6 × 107 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawirska-Olszańska, A.; Zaczyńska, E.; Czarny, A.; Kolniak-Ostek, J. Chemical Characteristics of Ethanol and Water Extracts of Black Alder (Alnus glutinosa L.) Acorns and Their Antibacterial, Anti-Fungal and Antitumor Properties. Molecules 2022, 27, 2804. https://doi.org/10.3390/molecules27092804
Nawirska-Olszańska A, Zaczyńska E, Czarny A, Kolniak-Ostek J. Chemical Characteristics of Ethanol and Water Extracts of Black Alder (Alnus glutinosa L.) Acorns and Their Antibacterial, Anti-Fungal and Antitumor Properties. Molecules. 2022; 27(9):2804. https://doi.org/10.3390/molecules27092804
Chicago/Turabian StyleNawirska-Olszańska, Agnieszka, Ewa Zaczyńska, Anna Czarny, and Joanna Kolniak-Ostek. 2022. "Chemical Characteristics of Ethanol and Water Extracts of Black Alder (Alnus glutinosa L.) Acorns and Their Antibacterial, Anti-Fungal and Antitumor Properties" Molecules 27, no. 9: 2804. https://doi.org/10.3390/molecules27092804
APA StyleNawirska-Olszańska, A., Zaczyńska, E., Czarny, A., & Kolniak-Ostek, J. (2022). Chemical Characteristics of Ethanol and Water Extracts of Black Alder (Alnus glutinosa L.) Acorns and Their Antibacterial, Anti-Fungal and Antitumor Properties. Molecules, 27(9), 2804. https://doi.org/10.3390/molecules27092804