Nanoformulation of Polyphenol Curcumin Enhances Cisplatin-Induced Apoptosis in Drug-Resistant MDA-MB-231 Breast Cancer Cells
Abstract
:1. Introduction
2. Results
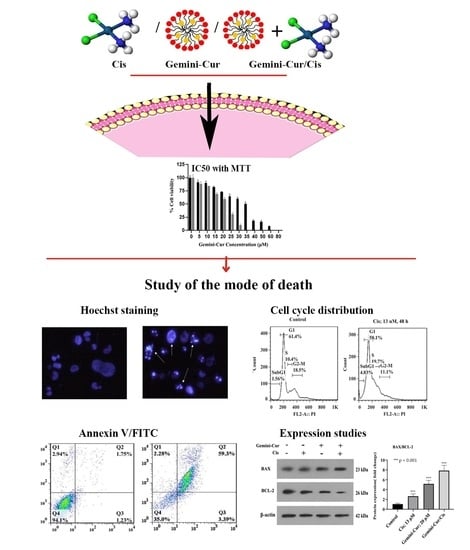
2.1. Gemini-Cur and Cisplatin Have a Synergistic Toxic Effect on MDA-MB-231 Cells
2.2. Morphological Visualization of Apoptosis
2.3. Gemini-Cur/Cis Modulates Cell Cycle Distribution in MDA-MB-231 Cells
2.4. Annexin V-FITC/PI Assay Confirmed Apoptosis in MDA-MB-231 Treated Cells
2.5. Expression of BAX, BCL-2 Genes Are Modulated in Treated Cells
3. Discussion
4. Materials and Methods
4.1. Reagents and Cell Culture
4.2. Cell Viability Assay
4.3. Analysis of Synergistic Cytotoxicity
4.4. Visualization of Apoptosis by Hoechst Staining
4.5. Cell Cycle Analysis by Flow Cytometry
4.6. Annexin V FITC/PI Assay
4.7. Expression Studies by Real-Time PCR and Western Blotting
4.7.1. Real-Time PCR
4.7.2. Western Blotting
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Baranova, A.; Krasnoselskyi, M.; Starikov, V.; Kartashov, S.; Zhulkevych, I.; Vlasenko, V.; Oleshko, K.; Bilodid, O.; Sadchikova, M.; Vinnyk, Y. Triple-negative breast cancer: Current treatment strategies and factors of negative prognosis. J. Med. Life 2022, 15, 153–161. [Google Scholar] [CrossRef]
- Ke, L.; Li, Z.; Fan, X.; Loh, X.J.; Cheng, H.; Wu, Y.-l.; Li, Z. Cyclodextrin-Based Hybrid Polymeric Complex to Overcome Dual Drug Resistance Mechanisms for Cancer Therapy. Polymers 2021, 13, 1254. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Makovec, T. Cisplatin and beyond: Molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol. Oncol. 2019, 53, 148–158. [Google Scholar] [CrossRef]
- Panda, A.K.; Chakraborty, D.; Sarkar, I.; Khan, T.; Sa, G. New insights into therapeutic activity and anticancer properties of curcumin. J. Exp. Pharmacol. 2017, 9, 31–45. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving Curcumin Bioavailability: Current Strategies and Future Perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef]
- Souto, E.B.; Silva, G.F.; Dias-Ferreira, J.; Zielinska, A.; Ventura, F.; Durazzo, A.; Lucarini, M.; Novellino, E.; Santini, A. Nanopharmaceutics: Part II-Production Scales and Clinically Compliant Production Methods. Nanomaterials 2020, 10, 455. [Google Scholar] [CrossRef]
- Babaei, E.; Sadeghizadeh, M.; Hassan, Z.M.; Feizi, M.A.H.; Najafi, F.; Hashemi, S.M. Dendrosomal curcumin significantly suppresses cancer cell proliferation in vitro and in vivo. Int. Immunopharmacol. 2012, 12, 226–234. [Google Scholar] [CrossRef]
- Zibaei, Z.; Babaei, E.; Rezaie Nezhad Zamani, A.; Rahbarghazi, R.; Azeez, H.J. Curcumin-enriched Gemini surfactant nanoparticles exhibited tumoricidal effects on human 3D spheroid HT-29 cells in vitro. Cancer Nano 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Hussain, Y.; Islam, L.; Khan, H.; Filosa, R.; Aschner, M.; Javed, S. Curcumin-cisplatin chemotherapy: A novel strategy in promoting chemotherapy efficacy and reducing side effects. Phytother. Res. 2021, 35, 6514–6529. [Google Scholar] [CrossRef]
- Zou, J.; Zhu, L.; Jiang, X.; Wang, Y.; Wang, Y.; Wang, X.; Chen, B. Curcumin increases breast cancer cell sensitivity to cisplatin by decreasing FEN1 expression. Oncotarget 2018, 9, 11268–11278. [Google Scholar] [CrossRef]
- Kang, J.H.; Kang, H.S.; Kim, I.K.; Lee, H.Y.; Ha, J.H.; Yeo, C.D.; Kang, H.H.; Moon, H.S.; Lee, S.H. Curcumin sensitizes human lung cancer cells to apoptosis and metastasis synergistically combined with carboplatin. Exp. Biol. Med. 2015, 240, 1416–1425. [Google Scholar] [CrossRef]
- Notarbartolo, M.; Poma, P.; Perri, D.; Dusonchet, L.; Cervello, M.; D’Alessandro, N. Antitumor effects of curcumin, alone or in combination with cisplatin or doxorubicin, on human hepatic cancer cells. Analysis of their possible relationship to changes in NF-kB activation levels and in IAP gene expression. Cancer Lett. 2005, 224, 53–65. [Google Scholar] [CrossRef]
- Montopoli, M.; Ragazzi, E.; Froldi, G.; Caparrotta, L. Cell-cycle inhibition and apoptosis induced by curcumin and cisplatin or oxaliplatin in human ovarian carcinoma cells. Cell Prolif. 2009, 42, 195–206. [Google Scholar] [CrossRef]
- Zheng, Z.H.; You, H.Y.; Feng, Y.J.; Zhang, Z.T. LncRNA KCNQ1OT1 is a key factor in the reversal effect of curcumin on cisplatin resistance in the colorectal cancer cells. Mol. Cell Biochem. 2021, 476, 2575–2585. [Google Scholar] [CrossRef]
- Karimpour, M.; Feizi, M.A.H.; Mahdavi, M.; Krammer, B.; Verwanger, T.; Najafi, F.; Babaei, E. Development of curcumin-loaded gemini surfactant nanoparticles: Synthesis, characterization and evaluation of anticancer activity against human breast cancer cell lines. Phytomedicine 2019, 57, 183–190. [Google Scholar] [CrossRef]
- Balic, M.; Thomssen, C.; Würstlein, R.; Gnant, M.; Harbeck, N. St. Gallen/Vienna 2019: A Brief Summary of the Consensus Discussion on the Optimal Primary Breast Cancer Treatment. Breast Care 2019, 14, 103–110. [Google Scholar] [CrossRef]
- Chalakur-Ramireddy, N.K.R.; Pakala, S.B. Combined drug therapeutic strategies for the effective treatment of Triple Negative Breast Cancer. Biosci. Rep. 2018, 38, BSR20171357. [Google Scholar] [CrossRef]
- Shehzad, A.; Lee, J.; Huh, T.L.; Lee, Y.S. Curcumin induces apoptosis in human colorectal carcinoma (HCT-15) cells by regulating expression of Prp4 and p53. Mol. Cells. 2013, 35, 526–532. [Google Scholar] [CrossRef]
- Lopes-Rodrigues, V.; Sousa, E.; Vasconcelos, M.H. Curcumin as a Modulator of P-Glycoprotein in Cancer: Challenges and Perspectives. Pharmaceuticals 2016, 9, 71. [Google Scholar] [CrossRef]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]







| Genes | Primers | Size (bp) |
|---|---|---|
| β-actin | F: 5′-TGCCCATCTACGAGGGGTATG-3′ R: 5′-CTCCTTAATGTCACGCACGATTTC-3′ | 155 |
| BAX | F: 5′-GCAAACTGGTGCTCAAGG-3′ R: 5′-ACTCCCGCCACAAAGA-3′ | 236 |
| BCL-2 | F: 5′-TGGGAAGTTTCAAATCAGC-3′ R: 5′-GCATTCTTGGACGAGGG-3′ | 298 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karami, P.; Othman, G.; Housein, Z.; Salihi, A.; Hosseinpour Feizi, M.A.; Azeez, H.J.; Babaei, E. Nanoformulation of Polyphenol Curcumin Enhances Cisplatin-Induced Apoptosis in Drug-Resistant MDA-MB-231 Breast Cancer Cells. Molecules 2022, 27, 2917. https://doi.org/10.3390/molecules27092917
Karami P, Othman G, Housein Z, Salihi A, Hosseinpour Feizi MA, Azeez HJ, Babaei E. Nanoformulation of Polyphenol Curcumin Enhances Cisplatin-Induced Apoptosis in Drug-Resistant MDA-MB-231 Breast Cancer Cells. Molecules. 2022; 27(9):2917. https://doi.org/10.3390/molecules27092917
Chicago/Turabian StyleKarami, Parastoo, Goran Othman, Zjwan Housein, Abbas Salihi, Mohammad Ali Hosseinpour Feizi, Hewa Jalal Azeez, and Esmaeil Babaei. 2022. "Nanoformulation of Polyphenol Curcumin Enhances Cisplatin-Induced Apoptosis in Drug-Resistant MDA-MB-231 Breast Cancer Cells" Molecules 27, no. 9: 2917. https://doi.org/10.3390/molecules27092917
APA StyleKarami, P., Othman, G., Housein, Z., Salihi, A., Hosseinpour Feizi, M. A., Azeez, H. J., & Babaei, E. (2022). Nanoformulation of Polyphenol Curcumin Enhances Cisplatin-Induced Apoptosis in Drug-Resistant MDA-MB-231 Breast Cancer Cells. Molecules, 27(9), 2917. https://doi.org/10.3390/molecules27092917