Ultra-Thin Wrinkled Carbon Sheet as an Anode Material of High-Power-Density Potassium-Ion Batteries
Abstract
:1. Introduction
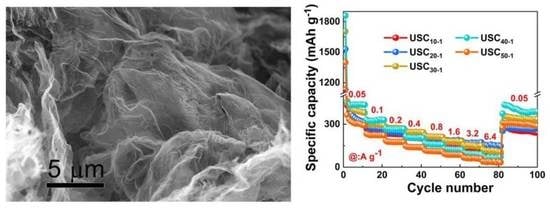
2. Results and Discussion
3. Experimental Section
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodenough, J.-B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.; Luo, W.; Ji, X. Carbon Electrodes for K-Ion Batteries. J. Am. Chem. Soc. 2015, 37, 11566–11569. [Google Scholar] [CrossRef] [PubMed]
- Alvin, S.; Cahyadi, H.-S.; Hwang, J.; Chang, W.; Kwak, S.-K. Revealing the Intercalation Mechanisms of Lithium, Sodium, and Potassium in Hard Carbon. Adv. Energy Mater. 2020, 10, 2000283. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, Y.; Meng, X.; Cao, W.; Liu, C.; Huang, Q.; Guo, Z. The impact of electrode with carbon materials on safety performance of lithium-ion batteries: A review. Carbon 2022, 191, 448–470. [Google Scholar] [CrossRef]
- Wang, G.; Yu, M.; Feng, X. Carbon materials for ion-intercalation involved rechargeable battery technologies. Chem. Soc. Rev. 2021, 50, 2388–2443. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Lu, S.; Xiang, Y. Carbon Anode Materials: A Detailed Comparison between Na-ion and K-ion Batteries. Adv. Energy Mater. 2021, 11, 2003640. [Google Scholar] [CrossRef]
- Hou, H.; Qiu, X.; Wei, W.; Zhang, Y.; Ji, X. Carbon Anode Materials for Advanced Sodium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1602898. [Google Scholar] [CrossRef]
- Yao, X.; Ke, Y.; Ren, W.; Wang, X.; Xiong, F.; Yang, W.; Mai, L. Defect-rich soft carbon porous nanosheets for fast and high-capacity sodium-ion storage. Adv. Energy Mater. 2018, 9, 1803260. [Google Scholar] [CrossRef]
- Ma, G.-Y.; Huang, K.-S.; Ma, J.-S.; Ju, Z.-C.; Xing, Z.; Zhuang, Q.-C. Phosphorus and oxygen dual-doped graphene as superior anode material for room-temperature potassium-ion batteries. J. Mater. Chem. A 2017, 5, 7854–7861. [Google Scholar] [CrossRef]
- Qu, D.-Y.; Zhao, B.-L.; Song, Z.-Q.; Wang, D.-D.; Kong, H.-J.; Gan, S.-Y.; Ma, Y.-M.; Dong, X.-D.; Han, D.-X.; Niu, L. Two-dimensional N/O co-doped porous turbostratic carbon nanomeshes with expanded interlayer spacing as host material for potassium/lithium half/full batteries. J. Mater. Chem. A 2021, 9, 25094–25103. [Google Scholar] [CrossRef]
- Zhong, Y.-L.; Dai, W.-X.; Liu, D.; Wang, W.; Wang, L.-T.; Xie, J.P.; Hong, G. Nitrogen and Fluorine Dual Doping of Soft Carbon Nanofibers as Advanced Anode for Potassium Ion Batteries. Small 2021, 17, 2101576. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, Y.-L.; Xing, Z.; Lam, C.-W.-K.; Pang, S.-S.; Zhang, W.; Ju, Z.-C. Advanced Carbon-Based Anodes for Potassium-Ion Batteries. Adv. Energy Mater. 2019, 9, 1900343. [Google Scholar] [CrossRef]
- Zheng, J.-F.; Wu, Y.-J.; Sun, Y.-J.; Rong, J.-H.; Li, H.-Y.; Niu, L. Advanced Anode Materials of Potassium Ion Batteries: From Zero Dimension to Three Dimensions. Nano-Micro Lett. 2021, 13, 12. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.-X.; Xu, Y.-S.; Meng, Q.-S.; Gao, J.-C.; Sun, Y.-G.; Hu, Y.-S.; Chang, B.-B.; Liu, C.-T.; Cao, A.-M. Pitch-Derived Soft Carbon as Stable Anode Material for Potassium Ion Batteries. Adv. Mater. 2020, 32, 2000505. [Google Scholar] [CrossRef]
- Wang, X.-P.; Han, K.; Qin, D.-D.; Li, Q.; Wang, C.-Y.; Niua, C.-J.; Mai, L.-Q. Polycrystalline soft carbon semi-hollow microrods as anode for advanced K-ion full batteries. Nanoscale 2017, 9, 18216–18222. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Yin, W.; Hirano, S.-I. Carbon-coated graphene/antimony composite with a sandwich-like structure for enhanced sodium storage. J. Mater. Chem. A 2017, 5, 20623–20630. [Google Scholar] [CrossRef]
- Wang, M.-Y.; Zhu, Y.-Y.; Zhang, Y.; Yang, T.; Duan, J.-Y.; Wang, C.-Y. Cost-effective hard–soft carbon composite anodes with promising potassium ions storage performance. Electrochim. Acta 2021, 368, 137649. [Google Scholar] [CrossRef]
- Hu, J.; Xie, Y.; Yin, M.; Zhang, Z. Nitrogen doping and graphitization tuning coupled hard carbon for superior potassium-ion storage. J. Energy Chem. 2020, 49, 327–334. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, C.-G.; Zhou, M.; Fu, Q.; Zhao, C.-X.; Wu, M.-H.; Lei, Y. Highly nitrogen doped carbon nanofibers with superior rate capability and cyclability for potassium ion batteries. Nat. Commun. 2018, 9, 1720. [Google Scholar] [CrossRef]
- Hou, H.-S.; Shao, L.-D.; Zhang, Y.; Zou, G.-Q.; Chen, J.; Ji, X.-B. Large-Area Carbon Nanosheets Doped with Phosphorus: A High-Performance Anode Material for Sodium-Ion Batteries. Adv. Sci. 2017, 4, 1600243. [Google Scholar] [CrossRef]
- Sun, F.; Wang, H.; Qu, Z.-B.; Wang, K.-F.; Wang, L.-J.; Gao, J.-H.; Gao, J.-M.; Liu, S.-Q.; Lu, Y.-F. Carboxyl-Dominant Oxygen Rich Carbon for Improved Sodium Ion Storage: Synergistic Enhancement of Adsorption and Intercalation Mechanisms. Adv. Energy Mater. 2021, 11, 2002981. [Google Scholar] [CrossRef]
- Zhang, K.; He, Q.; Xiong, F.-Y.; Zhou, J.-P.; Zhao, Y.; Mai, L.-Q.; Zhang, L.-N. Active sites enriched hard carbon porous nanobelts for stable and high-capacity potassium-ion storage. Nano Energy 2020, 77, 105018. [Google Scholar] [CrossRef]
- Hong, Z.-S.; Zhen, Y.-C.; Ruan, Y.-R.; Kang, M.-L.; Zhou, K.-Q.; Zhang, J.-M.; Huang, Z.-G.; Wei, M.-D. Rational Design and General Synthesis of S-Doped Hard Carbon with Tunable Doping Sites toward Excellent Na-Ion Storage Performance. Adv. Mater. 2018, 30, 1802035. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zheng, S.; Wang, L.; Zhang, X. Metal-Free Chemoselective Hydrogenation of Nitroarenes by N-Doped Carbon Nanotubes via In Situ Polymerization of Pyrrole. ACS Omega 2020, 5, 7519–7528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jian, Z.; Xing, Z.; Bommier, C.; Li, Z.; Ji, X. Hard Carbon Microspheres: Potassium-Ion Anode Versus Sodium-Ion Anode. Adv. Energy Mater. 2016, 6, 1501874. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Xiang, Y.-G.; Zou, G.-Q.; Hou, H.-S.; Deng, W.-T.; Ji, X.-B. High Sulfur-Doped Hard Carbon with Advanced Potassium Storage Capacity via a Molten Salt Method. ACS Appl. Mater. Interfaces 2020, 12, 30431–30437. [Google Scholar] [CrossRef]
- Share, K.; Cohn, A.P.; Carter, R.; Rogers, B.; Pint, C.L. Role of Nitrogen-Doped Graphene for Improved High-Capacity Potassium Ion Battery Anodes. ACS Nano 2016, 10, 9738–9744. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, H.-D.; Wu, L.; Zhou, W.-B.; He, L.; Wang, W.-G.; Yan, W.-Q.; Huang, Q.-H.; Fu, L.-J.; Wu, Y.-P. A Large Scalable and Low-Cost Sulfur/Nitrogen Dual-Doped Hard Carbon as the Negative Electrode Material for High-Performance Potassium-Ion Batteries. Adv. Energy Mater. 2019, 9, 1901379. [Google Scholar] [CrossRef]
- Jian, Z.-L.; Hwang, S.-Y.; Li, Z.-F.; Hernandez, A.-S.; Wang, X.-F.; Xing, Z.-Y.; Su, D.; Ji, X.-L. Hard–Soft Composite Carbon as a Long-Cycling and High-Rate Anode for Potassium-Ion Batteries. Adv. Funct. Mater. 2017, 27, 1700324. [Google Scholar] [CrossRef]
- Fleischmann, S.; Mitchell, J.-B.; Wang, R.-C.; Zhan, C.; Jiang, D.-E.; Presser, V.; Augustyn, V. Pseudocapacitance: From Fundamental Understanding to High Power Energy Storage Materials. Chem. Rev. 2020, 120, 6738–6782. [Google Scholar] [CrossRef]
- Fang, G.-Z.; Wu, Z.-X.; Zhou, J.; Zhu, C.-Y.; Cao, X.-X.; Lin, T.-Q.; Chen, Y.-M.; Wang, C.; Pan, A.-Q.; Liang, S.-Q. Observation of Pseudocapacitive Effect and Fast Ion Diffusion in Bimetallic Sulfides as an Advanced Sodium-Ion Battery Anode. Adv. Energy Mater. 2018, 8, 1703155. [Google Scholar] [CrossRef]
- Augustyn, V.; Come, J.; Lowe, M.-A.; Kim, J.-W.; Taberna, P.-L.; Tolbert, S.-H.; Abruna, H.-D.; Simon, P.; Dunm, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Sun, S.; Yang, M.; Zhao, X. Typha-derived hard carbon for high-performance sodium ion storage. J. Alloys Compd. 2019, 784, 1290–1296. [Google Scholar] [CrossRef]
- Pei, L.-Y.; Yang, L.-T.; Cao, H.-L.; Liu, P.-Z.; Zhao, M.; Xu, B.-S.; Guo, J.-J. Cost-effective and renewable paper derived hard carbon microfibers as superior anode for sodium-ion batteries. Electrochim. Acta 2020, 364, 137313. [Google Scholar] [CrossRef]
- Chao, H.-X.; Qin, H.-Q.; Zhang, M.-D.; Huang, Y.-C.; Cao, L.-F.; Guo, H.-L.; Wang, K.; Teng, X.-L.; Cheng, J.-K.; Lu, Y.-K.; et al. Boosting the Pseudocapacitive and High Mass-Loaded Lithium/Sodium Storage through Bonding Polyoxometalate Nanoparticles on MXene Nanosheets. Adv. Funct. Mater. 2021, 31, 2007636. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, B.; Li, X.; Pan, L.; Xu, H.; Duan, H.; Wu, Q.; Yin, B.; He, H. Ultra-Thin Wrinkled Carbon Sheet as an Anode Material of High-Power-Density Potassium-Ion Batteries. Molecules 2022, 27, 2973. https://doi.org/10.3390/molecules27092973
Cheng B, Li X, Pan L, Xu H, Duan H, Wu Q, Yin B, He H. Ultra-Thin Wrinkled Carbon Sheet as an Anode Material of High-Power-Density Potassium-Ion Batteries. Molecules. 2022; 27(9):2973. https://doi.org/10.3390/molecules27092973
Chicago/Turabian StyleCheng, Boshi, Xing Li, Linhai Pan, Hongqiang Xu, Haojie Duan, Qian Wu, Bo Yin, and Haiyong He. 2022. "Ultra-Thin Wrinkled Carbon Sheet as an Anode Material of High-Power-Density Potassium-Ion Batteries" Molecules 27, no. 9: 2973. https://doi.org/10.3390/molecules27092973
APA StyleCheng, B., Li, X., Pan, L., Xu, H., Duan, H., Wu, Q., Yin, B., & He, H. (2022). Ultra-Thin Wrinkled Carbon Sheet as an Anode Material of High-Power-Density Potassium-Ion Batteries. Molecules, 27(9), 2973. https://doi.org/10.3390/molecules27092973