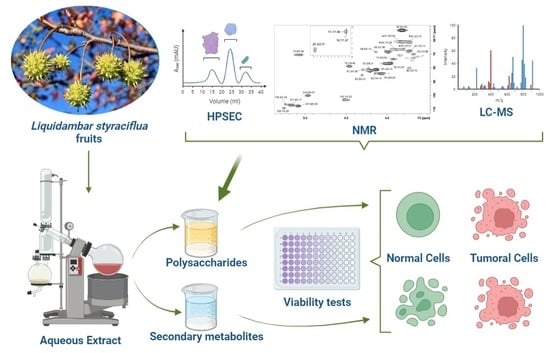
Chemical Evaluation of Liquidambar styraciflua L. Fruits Extracts and Their Potential as Anticancer Drugs
Abstract
:1. Introduction
2. Results and Discussion
2.1. Carbohydrates Present in L. styraciflua Extracts
2.2. Secondary Metabolites of L. styraciflua Extracts
2.3. Antioxidant and Antitumoral Effects of L. styraciflua Extracts
3. Material and Methods
3.1. Plant Material
3.2. Extraction Procedures
3.3. Determination of Phenolic Compounds, Proteins, Carbohydrates, and Uronic Acid
3.4. Reduction of Uronic Acids
3.5. Monosaccharide Analysis
3.6. Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis
3.7. Determination of Homogeneity and Relative Molecular Weight
3.8. Nuclear Magnetic Resonance Spectroscopy
3.9. Scavenging Activity of L. styraciflua Extracts Measured by DPPH Assay
3.10. Cell Culture
3.11. Cell Treatment with L. styraciflua Extracts
3.12. Cell Viability Assay
3.13. Cell Cycle
3.14. Apoptosis and Necrosis Assessment
3.15. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Eid, H.H.; Labib, R.M.; Hamid, N.S.A.; Hamed, M.A.; Ross, S.A. Hepatoprotective and antioxidant polyphenols from a standardized methanolic extract of the leaves of Liquidambar styraciflua L. Bull. Fac. Pharm. Cairo Univ. 2015, 53, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Mancarz, G.F.F.; Lobo, A.C.P.; Baril, M.B.; Franco, F.A.; Nakashima, T. Antimicrobial and Antioxidant Activity of the Leaves, Bark and Stems of Liquidambar styraciflua L. (Altingiaceae). Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 306–317. [Google Scholar] [CrossRef] [Green Version]
- Rashed, K.N.Z.; Sucupira, A.C.C.; Ferreira, P.M.P.; Feitosa, C.M. Phytoconstituents and evaluation of acetylcholinesterase inhibition by methanol extract of Liquidambar styraciflua (L.) aerial parts. J. Appl. Pharm. 2014, 6, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Yang, P.; Newman, R.A. Sweet Gum Fruit Extract as a Therapeutic Agent. U.S. Patent 6,738,537, 21 May 2008. [Google Scholar]
- El-Readi, M.Z.; Eid, H.H.; Ashour, M.L.; Eid, S.Y.; Labib, R.M.; Sporer, F.; Wink, M. Variations of the chemical composition and bioactivity of essential oils from leaves and stems of Liquidambar styraciflua (Altingiaceae). J. Pharm. Pharmacol. 2013, 65, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Mancarz, G.F.F.; Laba, L.C.; Silva, E.C.P.; Prado, M.R.M.; Souza, L.M.; Souza, D.; Nakashima, T.; Mello, R.G. Liquidambar styraciflua L.: A new potential source for therapeutic uses. J. Pharm. Biomed. Anal. 2019, 174, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yue, R.Q.; Liu, J.; Ho, H.M.; Yi, T.; Chen, H.B.; Han, Q.B. Structural diversity requires individual optimization of ethanol concentration in polysaccharide precipitation. Int. J. Biol. Macromol. 2014, 67, 205–209. [Google Scholar] [CrossRef] [Green Version]
- Martin, E.; Duke, J.; Pelkki, M.; Clausen, E.C.; Carrier, D.J. Sweetgum (Liquidambar styraciflua L.): Extraction of Shikimic acid coupled to dilute acid pretreatment. Appl. Biochem. Biotechnol. 2010, 162, 1660–1668. [Google Scholar] [CrossRef]
- Bufacchi, P.; Bizzo, W.A.; Buckeridge, M.S.; Franco-Jacome, D.L.; Grandis, A.; Cambler, A.B.; Krieger Filho, G.C. Thermal degradation of leaves from the Amazon rainforest litter considering non-structural, structural carbohydrates and lignin composition. Bioresour. Technol. Rep. 2020, 11, 100490. [Google Scholar] [CrossRef]
- Dibanda, R.F.; Akdowa, E.P.; Rani, A.; Tongwa, Q.M.; Mbofung, C.M. Effect of microwave blanching on antioxidant activity, phenolic compounds and browning behaviour of some fruit peelings. Food Chem. 2019, 302, 125308. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Widelska, G.; Wójtowicz, A.; Oniszczuk, T.; Wojtunik-Kulesza, K.; Dib, A.; Matwijczuk, A. Content of phenolic compounds and antioxidant activity of new gluten-free pasta with the addition of chestnut flour. Molecules 2019, 24, 2623. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Wang, K.; Pan, Y.; Wang, H.; Zhang, Y.; Lei, Q.; Zhu, Z.; Liang, M. Antioxidant activities of Liquidambar formosana Hance leaf extracts. Med. Chem. Res. 2010, 19, 166–176. [Google Scholar] [CrossRef]
- Terashima, M.; Kakuno, Y.; Kitano, N.; Matsuoka, C.; Murase, M.; Togo, N.; Matsumura, S. Antioxidant activity of flavonoids evaluated with myoglobin method. Plant Cell Rep. 2011, 31, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Ye, X.; Chantapakul, T.; Chen, S.; Zheng, J. Manosonication extraction of RG-I pectic polysaccharides from citrus waste: Optimization and kinetics analysis. Carbohydr. Polym. 2020, 235, 115982. [Google Scholar] [CrossRef]
- Feriani, A.; Tir, M.; Hamed, M.; Sila, A.; Nahdi, S.; Alwasel, S.; Tlili, N. Multidirectional insights on polysaccharides from Schinus terebinthifolius and Schinus molle fruits: Physicochemical and functional profiles, in vitro antioxidant, anti-genotoxicity, antidiabetic, and antihemolytic capacities, and in vivo anti-inflammatory and anti-nociceptive properties. Int. J. Biol. Macromol. 2020, 165, 2576–2587. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, T.; Hu, X.; Ren, G.; Zhang, H.; Wang, Z.; Wu, J. Structural, rheological properties and antioxidant activities of polysaccharides from mulberry fruits (Murus alba L.) based on different extraction techniques with superfine grinding pretreatment. Int. J. Biol. Macromol. 2021, 183, 1774–1783. [Google Scholar] [CrossRef]
- Zhang, J.; Song, Z.; Li, Y.; Zhang, S.; Bao, J.; Wang, H.; Guo, Y. Structural analysis and biological effects of a neutral polysaccharide from the fruits of Rosa laevigata. Carbohydr. Polym. 2021, 265, 118080. [Google Scholar] [CrossRef]
- Xu, J.; Wang, R.; Liu, J.; Cheng, H.; Peng, D.; Xing, L.; Yu, N. Determination of monosaccharides in Lycium barbarum fruit polysaccharide by an efficient UHPLC-QTRAP-MS/MS method. Phytochem. Anal. 2021, 32, 785–793. [Google Scholar] [CrossRef]
- Kytidou, K.; Artola, M.; Overkleeft, H.S.; Aerts, J.M.F.G. Plant glycosides and glycosidases: A treasure-trove for therapeutics. Front. Plant Sci. 2020, 11, 357. [Google Scholar] [CrossRef] [Green Version]
- Brecker, L.; Wicklein, D.; Moll, H.; Fuchs, E.C.; Becker, W.M.; Petersen, A. Structural and immunological properties of arabinogalactan polysaccharides from pollen of timothy grass (Phleum pratense L.). Carbohydr. Res. 2005, 340, 657–663. [Google Scholar] [CrossRef]
- Chaves, P.F.P.; Iacomini, M.; Cordeiro, L.M.C. Chemical characterization of fructooligosaccharides, inulin and structurally diverse polysaccharides from chamomile tea. Carbohydr. Polym. 2019, 214, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Fang, J.N. Structural elucidation of a new arabinogalactan from the leaves of Nerium indicum. Carbohydr. Res. 2001, 332, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Klosterhoff, R.; Bark, J.M.; Glänzel, N.M.; Iacomini, M.; Martinez, G.R.; Winnischofer, S.M.B.; Cordeiro, L.M.C. Structure and intracellular antioxidant activity of pectic polysaccharide from acerola (Malpighia emarginata). Int. J. Biol. Macromol. 2018, 106, 473–480. [Google Scholar] [CrossRef]
- Popov, S.V.; Ovodova, R.G.; Golovchenko, V.V.; Popova, G.Y.; Viatyasev, F.V.; Shashkov, A.S.; Ovodov, Y.S. Chemical composition and anti-inflammatory activity of a pectic polysaccharide isolated from sweet pepper using a simulated gastric medium. Food Chem. 2011, 124, 309–315. [Google Scholar] [CrossRef]
- Baky, M.H.; Badawy, M.T.; Bakr, A.F.; Hegazi, N.M.; Abdellatif, A.; Farag, M.A. Metabolome-based profiling of African baobab fruit (Adansonia digitata L.) using a multiplex approach of MS and NMR techniques in relation to its biological activity. RSC Adv. 2021, 11, 39680–39695. [Google Scholar] [CrossRef] [PubMed]
- Carlier, J.; Romeuf, L.; Guitton, J.; Priez-Barallon, C.; Bévalot, F.; Fanton, L.; Gaillard, Y. A validated method for quantifying atractyloside and carboxyatractyloside in blood by HPLC-HRMS/MS, a non-fatal case of intoxication with Atractylis gummifera L. J. Anal. Toxicol. 2014, 38, 619–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Qi, X.; Liu, Y.; Liu, Y.; Lv, X.; Sun, J.; Cai, Q. UPLC-MS/MS of Atractylenolide I, Atractylenolide II, Atractylenolide III, and Atractyloside A in rat plasma after oral administration of raw and wheat bran-processed Atractylodis Rhizoma. Molecules 2018, 23, 3234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karatoprak, G.S.; Akkol, E.K.; Genç, Y.; Bardakci, H.; Yücel, Ç.; Sobarzo-Sánchez, E. Combretastatins: An overview of structure, probable mechanisms of action and potential applications. Molecules 2020, 25, 2560. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Fukuda, Y.; Matsunaga, S.; Tanaka, R.; Yamori, T. New cytotoxic oleanane-type triterpenoids from the cones of Liquidambar styraciflua. J. Nat. Prod. 2004, 67, 1088–1093. [Google Scholar] [CrossRef]
- Yan, R.; Zhu, H.; Huang, P.; Zhang, W.; Hao, P.; Qu, Y. Liquidambaric acid inhibits Wnt/b-catenin signaling and colon cancer via targeting TNF receptor associated factor 2. Cell Rep. 2022, 38, 110319. [Google Scholar] [CrossRef]
- Lingbeck, J.M.; O′Bryan, C.A.; Martin, E.M.; Adams, J.P.; Crandall, P.G. Sweetgum: An ancient source of beneficial compounds with modern benefits. Pharmacogn. Rev. 2015, 9, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, W.; Shi, Y.; Wang, R.; Su, D.; Tang, M.; Liu, Y.; Li, Z. Antioxidant activity and healthy benefits of natural pigments in fruits: A review. Int. J. Mol. Sci. 2021, 22, 4945. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Saeed, M.E.M.; Mirghani, E.; Alim, A.; Yassin, Z.; Saeed, E.; Khalid, H.E.; Daak, S. Integration of phytochemicals and phytotherapy into cancer precision medicine. Oncotarget 2017, 8, 50284–50304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freire, P.F.; Peropadre, A.; Martín, J.M.P.; Herrero, O.; Hazen, M.J. An integrated cellular model to evaluate cytotoxic effects in mammalian cell lines. Toxicol. Vitr. 2009, 23, 1553–1558. [Google Scholar] [CrossRef]
- Zong, A.; Cao, H.; Wang, F. Anticancer polysaccharides from natural resources: A review of recent research. Carbohydr. Polym. 2012, 90, 1395–1410. [Google Scholar] [CrossRef] [PubMed]
- Nasir, N.N.; Sekar, M.; Fuloria, S.; Gan, S.H.; Rani, N.N.I.M.; Ravi, S.; Begum, M.Y.; Chidambaram, K.; Sathasivam, K.V.; Jeyabalan, S.; et al. Kirenol: A potential natural lead molecule for a new drug design, development, and therapy for inflammation. Molecules 2022, 27, 734. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity. Distributed through American National Standards Institute (ANSI): New York, NY, USA, 2009.
- Ramberg, J.E.; Nelson, E.D.; Sinnott, R.A. Immunomodulatory dietary polysaccharides: A systematic review of the literature. Nutr. J. 2010, 9, 54. [Google Scholar] [CrossRef] [Green Version]
- Corso, C.R.; Mulinari Turin de Oliveira, N.; Moura Cordeiro, L.; Sauruk da Silva, K.; da Silva Soczek, S.H.; Frota Rossato, V.; Fernandes, E.S.; Maria-Ferreira, D. Polysaccharides with Antitumor Effect in Breast Cancer: A Systematic Review of Non-Clinical Studies. Nutrients 2021, 13, 2008. [Google Scholar] [CrossRef]
- Tang, Z.; Zhou, C.; Cai, Y.; Tang, Y.; Sun, W.; Yao, H.; Zheng, T.; Chen, H.; Xiao, Y.; Shan, Z.; et al. Purification, characterization and antioxidant activities in vitro of polysaccharides from Amaranthus hybridus L. PeerJ 2020, 8, e9077. [Google Scholar] [CrossRef]
- Smiderle, F.R.; Baggio, C.H.; Borato, D.G.; Santana-Filho, A.P.; Sassaki, G.L.; Iacomini, M.; Van Griensven, L.J.L.D. Anti-inflammatory properties of the medicinal mushroom Cordyceps militaris might be related to its linear (1→3)-β-D-glucan. PloS ONE 2014, 9, e110266. [Google Scholar] [CrossRef]
- Pfeifer, L.; Baumann, A.; Petersen, L.M.; Höger, B.; Beitz, E.; Classen, B. Degraded Arabinogalactans and Their Binding Properties to Cancer-Associated Human Galectins. Int. J. Mol. Sci. 2021, 22, 4058. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yang, S.; Jin, L.; Hu, D.; Wu, Z.; Yang, S. Chemical constituents of the ethyl acetate extract of Belamcanda chinensis (L.) DC roots and their antitumor activities. Molecules 2012, 17, 6156–6169. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.I.; Savinova, O.V.; Nikolaeva, S.N.; Boreko, E.I.; Flekhter, O.B. Antiviral activity of betulin, betulinic and betulonic acids against some enveloped and non-enveloped viruses. Fitoterapia 2003, 74, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Sá, M.S.; Costa, J.F.O.; Kreettli, A.U.; Zalis, M.G.; Maia, G.L.A.; Sette, I.M.F.; Câmara, C.A.; Barbosa Filho, J.M.; Giulietti-Harley, A.M.; Santos, R.R.; et al. Antimalarial activity of betulinic acid and derivatives in vitro against Plasmodium falciparum and in vivo in P. berghei-infected mice. Parasitol. Res. 2009, 105, 275–279. [Google Scholar] [CrossRef]
- Shai, L.J.; McGaw, L.J.; Aderogba, M.A.; Mdee, L.K.; Eloff, J.N. Four pentacyclic triterpenoids with antifungal and antibacterial activity from Curtisia dentata (Burm.f) C.A. Sm. Leaves. J. Ethnopharmacol. 2008, 119, 238–244. [Google Scholar] [CrossRef]
- Stewart, M.J.; Steenkamp, V. The biochemistry and toxicity of atractyloside: A review. Ther. Drug Monit. 2000, 22, 641–649. [Google Scholar] [CrossRef]
- D’Arcy, M. Cell death. A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Filisetti-Cozzi, T.M.C.C.; Carpita, N.C. Measurement of uronic acids without interference from neutral sugars. Anal. Biochem. 1991, 197, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.L.; Conrad, H.E. Stoichiometric depolymerization. Biochemistry 1966, 11, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, L.K.S.; Vecchia, D.D.; Wendler, E.M.; Hocayen, P.A.S.; Lívero, F.A.R.; Stipp, M.C.; Barcaro, I.M.R.; Acco, A.; Andreatini, R. Quercetin reduces manic-like behavior and brain oxidative stress induced by paradoxical sleep deprivation in mice. Free. Radic. Biol. Med. 2016, 99, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]









| Fractions | Extract Yield (%) | Proteins (mg eq. BSA)/g Extract) | Carbohydrates (mg eq. Glc)/g Extract) | Phenolic Compounds (mg eq. GA)/g Extract) |
|---|---|---|---|---|
| EA | 7.60 | 14.2 ± 0.06 | 250.0 ± 0.01 | 100.7 ± 0.85 |
| P-EA | 3.07 | 33.0 ± 0.07 | 250.0 ± 0.03 | 113.1 ± 3.00 |
| S-EA | 3.32 | Tr. | 200.0 ± 0.01 | 298.4 ± 8.12 |
| Peak | Rt (min) | MS1 | MS2 | Tentative Identification | References |
|---|---|---|---|---|---|
| 1 | 0.55 | 169.0135 | 125.0240 | Gallic acid | - |
| 2 | 0.94 | 153.0192 | 109.0297 | Protocatechuic acid | - |
| 3 | 3.12 | 327.1081 | 147.0443 | Hydroxyphenyl propanoic acid glycoside | [26] |
| 4 | 4.33 | 447.0909 | 300.0308/301.0387 | Quercetin-rhamnoside | - |
| 5 | 5.08 | 483.1978/493.2280 | 315.1793 | Atractyloside A | [27,28] |
| 6 | 6.04 | 327.2181 | -- | N.I. | - |
| 7 | 6.41 | 329.2335 | -- | N.I. | - |
| 8 | 7.74 | 333.1346 | -- | Combretastatin | [29] |
| 9 | 9.87 | 469.3322 | -- | 6β-hydroxy betunolic acid | [30] |
| 10 | 10.53 | 453.3367 | -- | Liquidambaric acid (isomer 1) | [31] |
| 11 | 10.64 | 453.3369 | -- | Liquidambaric acid (isomer 2) | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozzobon, R.G.; Rutckeviski, R.; Carlotto, J.; Schneider, V.S.; Cordeiro, L.M.C.; Mancarz, G.F.F.; Souza, L.M.d.; Mello, R.G.; Smiderle, F.R. Chemical Evaluation of Liquidambar styraciflua L. Fruits Extracts and Their Potential as Anticancer Drugs. Molecules 2023, 28, 360. https://doi.org/10.3390/molecules28010360
Pozzobon RG, Rutckeviski R, Carlotto J, Schneider VS, Cordeiro LMC, Mancarz GFF, Souza LMd, Mello RG, Smiderle FR. Chemical Evaluation of Liquidambar styraciflua L. Fruits Extracts and Their Potential as Anticancer Drugs. Molecules. 2023; 28(1):360. https://doi.org/10.3390/molecules28010360
Chicago/Turabian StylePozzobon, Rafaela G., Renata Rutckeviski, Juliane Carlotto, Vanessa S. Schneider, Lucimara M. C. Cordeiro, Graziele Francine Franco Mancarz, Lauro M. de Souza, Rosiane Guetter Mello, and Fhernanda Ribeiro Smiderle. 2023. "Chemical Evaluation of Liquidambar styraciflua L. Fruits Extracts and Their Potential as Anticancer Drugs" Molecules 28, no. 1: 360. https://doi.org/10.3390/molecules28010360
APA StylePozzobon, R. G., Rutckeviski, R., Carlotto, J., Schneider, V. S., Cordeiro, L. M. C., Mancarz, G. F. F., Souza, L. M. d., Mello, R. G., & Smiderle, F. R. (2023). Chemical Evaluation of Liquidambar styraciflua L. Fruits Extracts and Their Potential as Anticancer Drugs. Molecules, 28(1), 360. https://doi.org/10.3390/molecules28010360