Bioactive Compounds in Extracts from the Agro-Industrial Waste of Mango
Abstract
:1. Introduction
2. Mango Production
3. Mango Commercial Varieties
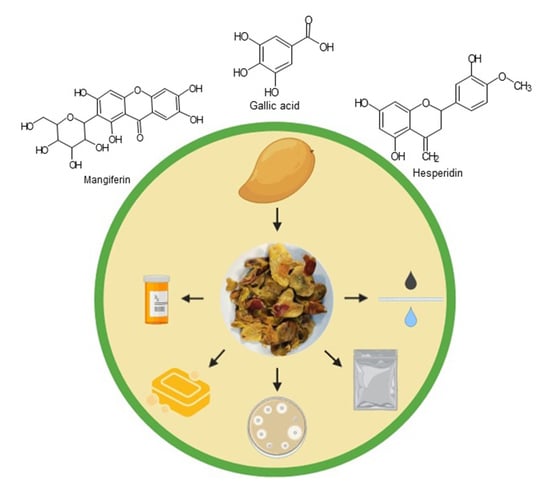
4. Mango Products and By-Products
5. Bioactive Compounds in Mango By-Products
6. Extraction of Bioactive Compounds
7. Mango Peel
7.1. Composition of Mango Peel
7.2. Extraction of Mango Peel Bioactive Compounds
7.3. Applications of Mango Peel Extracts
8. Mango Seed and Mango Seed Kernel
8.1. Composition of Mango Seed and Mango Seed Kernel
8.2. Extraction of Mango Kernel Bioactive Compounds
8.3. Applications of Bioactive Extracts from Mango Kernel
9. Perspectives
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS·+ | 2,2′-azonobis(3-ethylbenzothiazoline-6-sulphonate) |
| DAD | Diode Array |
| DPPH˙ | 2,2-diphenyl-1-picrylhydrazyl |
| FAO | Food and Agriculture Organization of the United Nations |
| FDA | Food and Drug Administration |
| FRAP | Ferric Ion Reducing Antioxidant Power |
| GAE | Gallic Acid Equivalents |
| GRAS | Generally Recognized as Safe |
| HPLC | High-Performance Liquid Chromatography |
| HPTLC | High-Performance Thin-Layer Chromatography |
| HVEDs | High-Voltage Electrical Discharges |
| IC50 | Half-Maximal Inhibitory Concentration |
| MS | Mass Spectrometry |
| PEFs | Pulsed Electric Fields |
| PVA | Polyvinyl Alcohol |
| ScCO2 | Super Critical Fluid Extraction with Carbon Dioxide |
| TEAC | Trolox Equivalence Antioxidant Capacity |
| TFCs | Total Flavonoid Compounds |
| TPCs | Total Phenolic Compounds |
| WE | Water Extraction |
References
- Asif, A.; Farooq, U.; Akram, K.; Hayat, Z.; Shafi, A.; Sarfraz, F.; Sidhu, M.A.I.; Rehman, H.; Aftab, S. Therapeutic Potentials of Bioactive Compounds from Mango Fruit. Trends Food Sci. Technol. 2016, 53, 102–112. [Google Scholar] [CrossRef]
- Coman, V.; Teleky, B.E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.F.; Vodnar, D.C. Bioactive Potential of Fruit and Vegetable Wastes. Adv. Food Nutr. Res. 2019, 91, 157–225. [Google Scholar] [CrossRef] [PubMed]
- López-Cobo, A.; Verardo, V.; Diaz-de-Cerio, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Gómez-Caravaca, A. Use of HPLC- and GC-QTOF to Determine Hydrophilic and Lipophilic Phenols in Mango Fruit (Mangifera indica L.) and Its by-Products. Food Res. Int. 2017, 100, 423–434. [Google Scholar] [CrossRef]
- Kaur, B.; Panesar, P.S.; Anal, A.K.; Ky, S.C. Recent Trends in the Management of Mango By-Products. Food Rev. Int. 2022, 00, 1–21. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Q.; Hu, M.; Gao, Z.; An, F.; Li, M.; Jiang, Y. Low-Temperature Conditioning Induces Chilling Tolerance in Stored Mango Fruit. Food Chem. 2017, 219, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Matheyambath, A.C.; Subramanian, J.; Paliyath, G. Mangoes. In Encyclopedia of Food and Health; Paul, B.C., Told, F.F., Eds.; Elsevier: Oxford, UK, 2016; pp. 641–645. [Google Scholar]
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillón, J.; Restrepo, B.; Guerrero, J.C. Chemical Composition of Mango (Mangifera indica L.) Fruit: Nutritional and Phytochemical Compounds. Front. Plant Sci. 2019, 10, 1073. [Google Scholar] [CrossRef]
- Siddiq, M.; Sogi, D.S.; Roidoung, S. Mango Processing and Processed Products. In Handbook of Mango Fruit: Production, Postharvest Science, Processing Technology and Nutrition; Siddiq, M., Brecht, J.K., Sidhu, J.S., Eds.; John Wiley & Sons Ltd.: Oxford, UK, 2017; pp. 195–216. [Google Scholar] [CrossRef]
- Okino-Delgado, C.H.; Francisco, L.F. Orange and Mango By-Products: Agro-Industrial Waste as Source of Bioactive Compounds and Botanical versus Commercial Description—A Review. Food Rev. Int. 2016, 32, 1–14. [Google Scholar] [CrossRef]
- Aggarwal, P.; Kaur, A.; Bhise, S. Value-Added Processing and Utilization of Mango by-Products. In Handbook of Mango Fruit: Production, Postharvest Science, Processing Technology and Nutrition; Siddiq, M., Brecht, J.K., Sidhu, J.S., Eds.; John Wiley & Sons Ltd.: Oxford, UK, 2017; pp. 279–293. [Google Scholar] [CrossRef]
- Wall-Medrano, A.; Olivas-Aguirre, F.J.; Ayala-Zavala, J.F.; Domínguez-Avila, J.A.; Gonzalez-Aguilar, G.A.; Herrera-Cazares, L.A.; Gaytan-Martinez, M. Health Benefits of Mango By-products. In Food Wastes and By-Products; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 159–191. [Google Scholar] [CrossRef]
- Jahurul, M.H.A.; Zaidul, I.S.M.; Ghafoor, K.; Al-Juhaimi, F.Y.; Nyam, K.-L.; Norulaini, N.A.N.; Sahena, F.; Mohd Omar, A.K. Mango (Mangifera indica L.) by-Products and Their Valuable Components: A Review. Food Chem. 2015, 183, 173–180. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO) Faostat Database. 2021. Available online: www.fao.org/faostat (accessed on 28 February 2021).
- Food and Agriculture Organization of the United Nations (FAO); Altendorf, S. Global Prospects for Major Tropical Fruits: Short-Term Outlook, Challenges and Opportunities in a Vibrant Global Marketplace; FAO: Rome, Italy, 2017. [Google Scholar]
- Yadav, A.S.; Pandey, D.C. Geographical Perspectives of Mango Production in India. Imp. J. Interdiscip. Res. 2016, 2, 257–265. [Google Scholar]
- Tiyayon, C.; Paull, R.E. Mango Production. In Handbook of Mango Fruit: Production, Postharvest Science, Processing Technology and Nutrition; Siddiq, M., Brecht, J.K., Sidhu, J.S., Eds.; John Wiley & Sons Ltd.: Oxford, UK, 2017; pp. 17–35. [Google Scholar] [CrossRef]
- Fitmawati, A.H.; Purwoko, D.B.S. Taxonomy of Cultivated Indonesian Mango in Practice. Indones. J. Agron. 2009, 37, 130–137. [Google Scholar]
- Liu, F.X.; Fu, S.F.; Bi, X.F.; Chen, F.; Liao, X.J.; Hu, X.S.; Wu, J.H. Physico-Chemical and Antioxidant Properties of Four Mango (Mangifera indica L.) Cultivars in China. Food Chem. 2013, 138, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Mellado-Vázquez, A.; Salazar-García, S.; Goenaga, R.; López-Jiménez, A. Survey of Fruit Nutrient Removal by Mango (Mangifera indica L.) Cultivars for the Export Market in Various Producing Regions of Mexico. Rev. Terra Latinoam. 2019, 37, 437–447. [Google Scholar] [CrossRef]
- Pereira, A.D.S.; Fontes-Sant’Ana, G.C.; Amaral, P.F.F. Mango Agro-Industrial Wastes for Lipase Production from Yarrowia lipolytica and the Potential of the Fermented Solid as a Biocatalyst. Food Bioprod. Process. 2019, 115, 68–77. [Google Scholar] [CrossRef]
- Badar, H.; Ahmad, B. Smallholders’ Constraints and Options for Participation in Mango Value Chains in Punjab, Pakistan. J. Agric. Res 2021, 59, 187–195. [Google Scholar]
- Serna-Cock, L.; García-Gonzales, E.; Torres-León, C. Agro-Industrial Potential of the Mango Peel Based on Its Nutritional and Functional Properties. Food Rev. Int. 2016, 32, 364–376. [Google Scholar] [CrossRef]
- Munafo, J.P.; Didzbalis, J.; Schnell, R.J.; Steinhaus, M. Insights into the Key Aroma Compounds in Mango (Mangifera indica L. ’Haden’) Fruits by Stable Isotope Dilution Quantitation and Aroma Simulation Experiments. J. Agric. Food Chem. 2016, 64, 4312–4318. [Google Scholar] [CrossRef]
- Morales-de la Peña, M.; Salinas-Roca, B.; Escobedo-Avellaneda, Z.; Martín-Belloso, O.; Welti-Chanes, J. Effect of High Hydrostatic Pressure and Temperature on Enzymatic Activity and Quality Attributes in Mango Puree Varieties (Cv. Tommy Atkins and Manila). Food Bioprocess Technol. 2018, 11, 1211–1221. [Google Scholar] [CrossRef]
- White, I.R.; Blake, R.S.; Taylor, A.J.; Monks, P.S. Metabolite Profiling of the Ripening of Mangoes Mangifera indica L. Cv. ‘Tommy Atkins’ by Real-Time Measurement of Volatile Organic Compounds. Metabolomics 2016, 12, 57. [Google Scholar] [CrossRef] [Green Version]
- Ernesto, D.B.; Omwamba, M.; Faraj, A.K.; Mahungu, S.M. Physico-Chemical Characterization of Keitt Mango and Cavendish Banana Fruits Produced in Mozambique. Food Nutr. Sci. 2018, 9, 556–571. [Google Scholar] [CrossRef]
- Guimarães, M.K.A.; Figueirêdo, M.R.F.; Queiroz, A.J.M. Foam-Mat Drying Kinetics of Keitt Mango Pulp. Rev. Caatinga 2017, 30, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Araiza, E.; Osuna, T.; Siller, J.; Contreras, L.; Sanchez, E. Postharvest Quality and Shelf-Life of Mango Cultivars Grown at Sinaloa, Mexico. Acta Hortic. 2005, 682, 1275–1280. [Google Scholar] [CrossRef]
- Léopold, K.; Tchoa, K.; Achille, N.; Hilaire, K. Correlation between Inflorescence Phenological Events of Mango (Mangifera indica Cv. Kent) and Some Climatic Parameters in Côte d’Ivoire. J. Exp. Agric. Int. 2018, 25, 1–12. [Google Scholar] [CrossRef]
- Oliveira Costa, L.; Lara Junior, J.M.; Correia da Costa, J.M.; Amorim Afonso, M.R.; Rodrigues, S.; Wurlitzer, N.J. Stability and Microstructure of Powdered Pulp of the Palmer Mango Obtained by the Process of Lyophilisation. Rev. Ciência Agronômica 2019, 50, 251–258. [Google Scholar] [CrossRef]
- Reddy, L.V.; Kim, Y.-M.; Wee, Y.-J. Rapid and Enhanced Liquefaction of Pulp from Mango (Mangifera indica L.) Cv. Totapuri Using Ultrasound-Assisted Enzyme Pretreatment. Processes 2020, 8, 718. [Google Scholar] [CrossRef]
- Deshpande, A.B.; Anamika, K.; Jha, V.; Chidley, H.G.; Oak, P.S.; Kadoo, N.Y.; Pujari, K.H.; Giri, A.P.; Gupta, V.S. Transcriptional Transitions in Alphonso Mango (Mangifera indica L.) during Fruit Development and Ripening Explain Its Distinct Aroma and Shelf Life Characteristics. Sci. Rep. 2017, 7, 8711. [Google Scholar] [CrossRef]
- Prashanth, K.V.H.; Baskaran, R.; Dhanyasri, E.B.; Rajashekaramurthy. Bioactive Chitosan Based Coatings: Functional Applications in Shelf Life Extension of Alphonso Mango-a Sweet Story. Pure Appl. Chem. 2016, 88, 853–863. [Google Scholar] [CrossRef]
- Quirós-Sauceda, A.E.; Oliver Chen, C.Y.; Blumberg, J.B.; Astiazaran-Garcia, H.; Wall-Medrano, A.; González-Aguilar, G.A. Processing ‘Ataulfo’ Mango into Juice Preserves the Bioavailability and Antioxidant Capacity of Its Phenolic Compounds. Nutrients 2017, 9, 1082. [Google Scholar] [CrossRef] [PubMed]
- Quirós-Sauceda, A.E.; Sañudo-Barajas, J.A.; Vélez-de la Rocha, R.; Domínguez-Avila, J.A.; Ayala-Zavala, J.F.; Villegas-Ochoa, M.A.; González-Aguilar, G.A. Effects of Ripening on the in Vitro Antioxidant Capacity and Bioaccessibility of Mango Cv. ‘Ataulfo’ Phenolics. J. Food Sci. Technol. 2019, 56, 2073–2082. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.M.R.; Barbosa, L.C.A.; Queiroz, J.H.; Knödler, M.; Schieber, A. Phenolic Compounds and Antioxidant Capacity of Brazilian Mango (Mangifera indica L.) Varieties. Food Chem. 2008, 110, 620–626. [Google Scholar] [CrossRef]
- Silva, D.F.P.; Siqueira, D.L.; Pereira, C.S.; Salomão, L.C.C.; Struiving, T.B. Caracterização de Frutos de 15 Cultivares de Mangueira Na Zona Da Mata Mineira. Rev. Ceres 2009, 56, 783–789. [Google Scholar]
- Alañón, M.E.; Palomo, I.; Rodríguez, L.; Fuentes, E.; Arráez-Román, D.; Segura-Carretero, A. Antiplatelet Activity of Natural Bioactive Extracts from Mango (Mangifera indica L.) and Its by-Products. Antioxidants 2019, 8, 517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Kady, T.; Abd El-Rahman, M.; Toliba, A.O.; Abo El-maty, S. Evaluation of Mango Seed Kernel Extract as Natural Occurring Phenolic Rich Antioxidant Compound. Bull. Natl. Nutr. Inst. 2017, 48, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Do Nascimento Oliveira, A.; De Almeida Paula, D.; de Oliveira, E.B.; Saraiva, S.H.; Stringheta, P.C.; Ramos, A.M. Optimization of Pectin Extraction from Ubá Mango Peel through Surface Response Methodology. Int. J. Biol. Macromol. 2018, 113, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Mugwagwa, L.R.; Chimphango, A.F.A. Box-Behnken Design Based Multi-Objective Optimisation of Sequential Extraction of Pectin and Anthocyanins from Mango Peels. Carbohydr. Polym. 2019, 219, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Bansal, M.; Pal, P.; Prajapati, P. Fruit Waste: Potential as a Functional Ingredient in Foods. In Emerging Technologies in Food Science; Thakur, M., Modi, V., Eds.; Springer Nature: Singapore, 2020; pp. 95–116. [Google Scholar]
- Rodríguez García, S.L.; Raghavan, V. Green Extraction Techniques from Fruit and Vegetable Waste to Obtain Bioactive Compounds—A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6446–6466. [Google Scholar] [CrossRef]
- Ekorong Akouan Anta, J.F.; Mbougueng, P.D.; Durand, E.; Baréa, B.; Villeneuve, P.; Ndjouenkeu, R. Model Development to Enhance the Solvent Extraction of Polyphenols from Mango Seed Kernel. J. Biol. Act. Prod. Nat. 2018, 8, 51–63. [Google Scholar] [CrossRef]
- del Pilar Sanchez-Camargo, A.; Gutiérrez, L.F.; Vargas, S.M.; Martinez-Correa, H.A.; Parada-Alfonso, F.; Narváez-Cuenca, C.E. Valorisation of Mango Peel: Proximate Composition, Supercritical Fluid Extraction of Carotenoids, and Application as an Antioxidant Additive for an Edible Oil. J. Supercrit. Fluids 2019, 152, 104574. [Google Scholar] [CrossRef]
- Kaur, B.; Srivast, P.P. Effect of Cryogenic Grinding on Chemical and Morphological Characteristics of Mango (Mangifera indica L.) Peel Powder. J. Food Process. Preserv. 2018, 42, e13583. [Google Scholar] [CrossRef]
- Oliver-Simancas, R.; Díaz-Maroto, M.C.; Pérez-Coello, M.S.; Alañón, M.E. Viability of Pre-Treatment Drying Methods on Mango Peel by-Products to Preserve Flavouring Active Compounds for Its Revalorisation. J. Food Eng. 2020, 279, 109953. [Google Scholar] [CrossRef]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Lebovka, N.; Vorobiev, E. Extraction Assisted by Pulsed Electric Energy as a Potential Tool for Green and Sustainable Recovery of Nutritionally Valuable Compounds from Mango Peels. Food Chem. 2016, 192, 842–848. [Google Scholar] [CrossRef]
- Safdar, M.N.; Kausar, T.; Nadeem, M. Comparison of Ultrasound and Maceration Techniques for the Extraction of Polyphenols from the Mango Peel. J. Food Process. Preserv. 2017, 41, e13028. [Google Scholar] [CrossRef]
- Lim, K.J.A.; Cabajar, A.A.; Lobarbio, C.F.Y.; Taboada, E.B.; Lacks, D.J. Extraction of Bioactive Compounds from Mango (Mangifera indica L. Var. Carabao) Seed Kernel with Ethanol–Water Binary Solvent Systems. J. Food Sci. Technol. 2019, 56, 2536–2544. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aty, A.M.; Salama, W.H.; Hamed, M.B.; Fahmy, A.S.; Mohamed, S.A. Phenolic-Antioxidant Capacity of Mango Seed Kernels: Therapeutic Effect against Viper Venoms. Brazilian J. Pharmacogn. 2018, 28, 594–601. [Google Scholar] [CrossRef]
- Nakpanich, N.; Chaiyana, W.; Leelapornpisid, P. Antioxidant Activities and Stability of Seed Kernel Extracts from Mango (Mangifera indica Linn.) Cultivated in Northern Thailand. Chiang Mai J. Sci. 2017, 44, 573–583. [Google Scholar]
- Correa, D.; Romero, B.; León, N. Extraction of Tannins from Creole Mango Seed (Mangifera indica L.) and Its Application as Tanning. J. Agro-Industry Sci. 2019, 1, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Ballesteros-Vivas, D.; Álvarez-Rivera, G.; Morantes, S.J.; del Pilar Sanchez-Camargo, A.; Ibáñez, E.; Parada-Alfonso, F.; Cifuentes, A. An Integrated Approach for the Valorization of Mango Seed Kernel: Efficient Extraction Solvent Selection, Phytochemical Profiling and Antiproliferative Activity Assessment. Food Res. Int. 2019, 126, 108616. [Google Scholar] [CrossRef] [PubMed]
- Torres-León, C.; Rojas, R.; Serna-Cock, L.; Belmares-Cerda, R.; Aguilar, C.N. Extraction of Antioxidants from Mango Seed Kernel: Optimization Assisted by Microwave. Food Bioprod. Process. 2017, 105, 188–196. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Castañeda-Valbuena, D.; Fernandez-Lafuente, R.; Berenguer-Murcia, Á.; Meza-Gordillo, R.; Gutiérrez, L.F.; Pacheco, N.; Cuevas-Bernardino, J.C.; Ayora-Talavera, T. Phenolic Compounds in Mango Fruit: A Review. J. Food Meas. Charact. 2022, 16, 619–636. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Quantitative Analysis, in Vitro Assessment of Bioavailability and Antioxidant Activity of Food Carotenoids-A Review. J. Food Compos. Anal. 2010, 23, 726–740. [Google Scholar] [CrossRef]
- Kiokias, S.; Proestos, C.; Varzakas, T. A Review of the Structure, Biosynthesis, Absorption of Carotenoids-Analysis and Properties of Their Common Natural Extracts. Curr. Res. Nutr. Food Sci. 2016, 4, 25–37. [Google Scholar] [CrossRef]
- Tesfaye, T. Valorisation of Mango Fruit By-Products: Physicochemical Characterisation and Future Prospect. Chem. Process Eng. Res. 2017, 50, 22–34. [Google Scholar]
- FDA. 21-CFR-184.1293. Code of Federal Regulations. Direct Food Substances Affirmed as Generally Recognized as Safe. Ethyl Al-cohol. 2022. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=%0A184.1293%0A (accessed on 20 November 2022).
- Kanatt, S.R.; Chawla, S.P. Shelf Life Extension of Chicken Packed in Active Film Developed with Mango Peel Extract. J. Food Saf. 2017, 38, e12385. [Google Scholar] [CrossRef] [Green Version]
- Umamahesh, K.; Ramesh, B.; Kumar, B.V.; Reddy, O.V.S. In Vitro Anti-Oxidant, Anti-Microbial and Anti-Inflammatory Activities of Five Indian Cultivars of Mango (Mangifera indica L.) Fruit Peel Extracts. J. HerbMed Pharmacol. 2019, 8, 238–247. [Google Scholar] [CrossRef]
- Gondi, M.; Prasada Rao, U.J.S. Ethanol Extract of Mango (Mangifera indica L.) Peel Inhibits α-Amylase and α-Glucosidase Activities, and Ameliorates Diabetes Related Biochemical Parameters in Streptozotocin (STZ)-Induced Diabetic Rats. J. Food Sci. Technol. 2015, 52, 7883–7893. [Google Scholar] [CrossRef] [Green Version]
- Espinosa-Espinosa, L.; Garduño-Siciliano, L.; Rodriguez-Canales, M.; Hernandez-Portilla, L.B.; Canales-Martinez, M.M.; Rodriguez-Monroy, M.A. The Wound-Healing Effect of Mango Peel Extract on Incision Wounds in a Murine Model. Molecules 2022, 27, 259. [Google Scholar] [CrossRef]
- Lizárraga-Velázquez, C.E.; Hernández, C.; González-Aguilar, G.A.; Heredia, J.B. Effect of Dietary Intake of Phenolic Compounds from Mango Peel Extract on Growth, Lipid Peroxidation and Antioxidant Enzyme Activities in Zebrafish (Danio Rerio). Lat. Am. J. Aquat. Res. 2019, 47, 602–611. [Google Scholar] [CrossRef] [Green Version]
- Manzoor, A.; Ahmad, S.; Yousuf, B. Effect of Bioactive-Rich Mango Peel Extract on Physicochemical, Antioxidant and Functional Characteristics of Chicken Sausage. Appl. Food Res. 2022, 2, 100183. [Google Scholar] [CrossRef]
- Torres-León, C.; Rojas, R.; Contreras-Esquivel, J.C.; Serna-Cock, L.; Belmares-Cerda, R.E.; Aguilar, C.N. Mango Seed: Functional and Nutritional Properties. Trends Food Sci. Technol. 2016, 55, 109–117. [Google Scholar] [CrossRef]
- Poul, S.S.; Bornare, D.T.; Babar, K.P. Nutritional and Functional Profiling of Mango Seed Powder and Its Suitability in Chakali. J. Pharmacogn. Phytochem. 2019, 8, 2460–2464. [Google Scholar]
- Sagar, A.; Singh, R.P. Biochemical Estimation of Nutritive Parameters in Waste Seed Kernel of Mango (Mangifera indica L.). Res. Environ. Life Sci. 2016, 9, 1358–1360. [Google Scholar] [CrossRef]
- Solís-Fuentes, J.A.; Durán-de-Bazúa, M.d.C. Mango Seed: Mango (Mangifera indica L.) Seeds and Its Fats. In Nuts and Seeds in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: London, UK, 2020; pp. 79–90. [Google Scholar]
- Villanueva, P.X.; Ávila, Y.C.; Dávila, L.R.; Méndez, J.J.; Murillo, W.A. Characterization and Use of Mangifera indica L. Seeds from Four Varieties. BioResources 2020, 15, 5264–5280. [Google Scholar] [CrossRef]
- Yadav, K.K.; Garg, N.; Verma, A.; Kumar, S.; Trivedi, M. Optimization and Extraction of Oil from Mango Seed Kernel (Mangifera indica). Indian J. Agric. Sci. 2017, 87, 91–94. [Google Scholar]
- López-Hernández, M.D.P.; Sandoval-Aldana, A.P.; Valencia-Montoya, J.A. Características Fisicoquímicas de La Grasa de Semilla de Veinte Cultivares de Mango (Mangifera indica L.) En Colombia. Rev. Bras. Frutic. 2016, 38, 10–21. [Google Scholar] [CrossRef]
- Dutra, E.D.; de Lima, T.A.; De Oliveira Souza, J.L.; Da Silva Aquino, K.A.; Da Silva Aquino, F.; Ramos, C.S.; Menezes, R.S.C. Characterization of Fat and Biodiesel from Mango Seeds Using 1H NMR Spectroscopy. Biomass Convers. Biorefinery 2017, 8, 135–141. [Google Scholar] [CrossRef]
- Jafarpour, A. Evaluation of Physicochemical Properties of Iranian Mango Seed Kernel Oil. Int. J. Biol. Sci. Res. 2019, 2, 143–149. [Google Scholar]
- Mas’Ud, F.; Mahendradatta, M.; Laga, A.; Zainal, Z. Optimization of Mango Seed Kernel Oil Extraction Using Response Surface Methodology. OCL Oilseeds Fats Crop. Lipids 2017, 24, D503. [Google Scholar] [CrossRef] [Green Version]
- Lim, K.J.A.; Cabajar, A.A.; Migallos, M.K.V.; Lobarbio, C.F.Y.; Taboada, E.B. Microencapsulation of Phenolic Compounds from Waste Mango Seed Kernel Extract by Spray Drying Technology. Nat. Environ. Pollut. Technol. 2019, 18, 765–775. [Google Scholar]
- Melo, P.E.F.; Silva, A.P.M.; Marques, F.P.; Ribeiro, P.R.V.; Souza Filho, M.D.S.M.; Brito, E.S.; Lima, J.R.; Azeredo, H.M.C. Antioxidant Films from Mango Kernel Components. Food Hydrocoll. 2019, 95, 487–495. [Google Scholar] [CrossRef]
- Maryam Adilah, Z.A.M.; Jamilah, B.; Nur Hanani, Z.A. Functional and Antioxidant Properties of Protein-Based Films Incorporated with Mango Kernel Extract for Active Packaging. Food Hydrocoll. 2018, 74, 207–218. [Google Scholar] [CrossRef]
- Maryam Adilah, Z.A.; Hanani, Z.A.N. Storage Stability of Soy Protein Isolate Films Incorporated with Mango Kernel Extract at Different Temperature. Food Hydrocoll. 2019, 87, 541–549. [Google Scholar] [CrossRef]
- Torres-León, C.; Vicente, A.A.; Flores-López, M.L.; Rojas, R.; Serna-Cock, L.; Alvarez-Pérez, O.B.; Aguilar, C.N. Edible Films and Coatings Based on Mango (Var. Ataulfo) by-Products to Improve Gas Transfer Rate of Peach. LWT 2018, 97, 624–631. [Google Scholar] [CrossRef] [Green Version]
- Widsten, P.; Mesic, B.B.; Cruz, C.D.; Fletcher, G.C.; Chycka, M.A. Inhibition of Foodborne Bacteria by Antibacterial Coatings Printed onto Food Packaging Films. J. Food Sci. Technol. 2017, 54, 2379–2386. [Google Scholar] [CrossRef] [PubMed]
- Seghosime, A.; Ebeigbe, A.B.; Awudza, J.A.M. Potential Use of Mangifera indica Seed Kernel and Citrus Aurantifolia Seed in Water Disinfection. Niger. J. Technol. 2017, 36, 1303–1310. [Google Scholar] [CrossRef] [Green Version]
- Torres-León, C.; Ramírez-Guzmán, N.; Ascacio-Valdés, J.; Serna-Cock, L.; dos Santos Correia, M.T.; Contreras-Esquivel, J.C.; Aguilar, C.N. Solid-State Fermentation with Aspergillus Niger to Enhance the Phenolic Contents and Antioxidative Activity of Mexican Mango Seed: A Promising Source of Natural Antioxidants. LWT 2019, 112, 108236. [Google Scholar] [CrossRef]
- Kukaittinun, K.; Satirapipathkul, C. Solid Lipid Nanoparticles from Active Compounds of Mango Seed Kernel Extract. Key Eng. Mater. 2016, 675–676, 65–68. [Google Scholar] [CrossRef]
- Dorta, E.; González, M.; Lobo, M.G.; Laich, F. Antifungal Activity of Mango Peel and Seed Extracts against Clinically Pathogenic and Food Spoilage Yeasts. Nat. Prod. Res. 2016, 30, 2598–2604. [Google Scholar] [CrossRef]
- Fresh Plaza. Global Overview Mangoes. 2021. Available online: https://www.freshplaza.com/article/9351797/global-overview-mangoes/%0A (accessed on 20 December 2022).
- Grand View Research. Frozen Fruits Market Size, Share & Trends Analysis Report By Product (Citrus, Tropical, Berries), By Distribution Channel (Online, Offline), By Region, And Segment Forecasts, 2020–2027. 2020. Available online: https://www.grandviewresearch.com/industry-analysis/frozen-fruits-market (accessed on 20 December 2022).
- Market Data Forecast. Dried Fruits Market Analysis by Type (Apricots, Dates, Figs, Peaches, Pears, Prunes, Raisins, Berries, and Others), Application (Confectioneries, Dairy Products, Bakery Products, Snacks & Bars, Desserts, Cereals, and Others) and Region (North America, Europe, Asia-Pacific, Middle East and Africa, Latin America)–Global Industry Analysis, Size, Growth, Investment and Forecasts 2022 to 2027. 2021. Available online: https://www.marketdataforecast.com/market-reports/dried-fruits-market (accessed on 20 December 2022).
- EMR Experts. Global Fruit Juice Market: By Type: 100% Fruit Juice, Nectars, Juice Drinks, Concentrates, Powdered Juice, Others; By Flavour: Orange, Apple, Mango, Mixed Fruit, Others; By Distribution Channel; Regional Analysis; Historical Market and Forecast (2016–2026). 2020. Available online: https://www.expertmarketresearch.com/reports/fruit-juice-market (accessed on 20 December 2022).
- Arora, A.; Banerjee, J.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F. Process Design and Techno-Economic Analysis of an Integrated Mango Processing Waste Biorefinery. Ind. Crops Prod. 2018, 116, 24–34. [Google Scholar] [CrossRef]
- Manhongo, T.T.; Chimphango, A.; Thornley, P.; Röder, M. An Economic Viability and Environmental Impact Assessment of Mango Processing Waste-Based Biorefineries for Co-Producing Bioenergy and Bioactive Compounds. Renew. Sustain. Energy Rev. 2021, 148, 111216. [Google Scholar] [CrossRef]
- Kee, L.K. Mango Seed Oil Skin-Protecting Detergent. Patent CN103275820B, 21 January 2015. [Google Scholar]
- Taboada, E.; Siacor, F.D. Preparation of Pectin and Polyphenolic Compositions from Mango Peels. Patent WO 2013/141723 Al, 26 September 2013. [Google Scholar]
- Karimi, J.; Hashemian, J. Paper Products and Methods of Making Paper Products with Antimicrobial Properties. Patent WO WO2017216602A1, 21 December 2017. [Google Scholar]
- Datamintelligence. Bioactive Ingredients Market. Datamintelligence. 2022. Available online: https://www.datamintelligence.com/research-report/bioactive-ingredients-market (accessed on 20 December 2022).
- García-Mahecha, M.; Carvajal-Millan, E.; Madera-Santana, T.J.; Lomelí-Ramírez, M.G.; Colín-Chávez, C.; Peralta, E.; Val-Félix, L.Á.; Soto-Valdez, H. Oportunidades Con Potencial Para El Aprovechamiento de Los Componentes Mayoritarios de Residuos Agroindustriales de Mango. In Tecnología, Ingeniería y Biotecnología de Alimentos de Origen Vegetal: Aprovechamiento de sus Subproductos; Montoya-Ballesteros, L.C., Tiznado-Hernández, M.E., Madera-Santana, T.J., Ayala-Zavala, J.F., González-Aguilar, G., Eds.; Editorial LIBERMEX-CIAD: Hermosillo, México, 2022; Chapter 1; pp. 17–32. [Google Scholar]



| By-Product | Method | Process or Extractant | Quantification Technique (Bioactive Compounds) | Reference |
|---|---|---|---|---|
| Peel | Cryogrinding | Moisture reduction | TFC and TPC | [46] |
| Drying | Moisture reduction | Volatile compounds | [47] | |
| PEFs and HVDEs | Water | TPC and proteins | [48] | |
| Pressurized Extraction | ScCO2 | Carotenoids | [45] | |
| Ultrasonication and Maceration | Ethanol and methanol | TPC (gallic and ferulic acid, epicatechin) | [49] | |
| Kernel | Solid—Liquid Extraction | Ethanol and water | TPC (rutin and penta-O-galloyl-β-D-glucose | [50] |
| Solid—Liquid Extraction | Methanol | TFC and TPC (hesperidin, cinnamic, tannic acid) | [51] | |
| Solid—Liquid Extraction | Methanol-acetone- water | TPC (chlorogenic acid, mangiferin, methyl gallate) | [44] | |
| Solid—Liquid Extraction | Ethanol | TPC (gallic acid) | [52] | |
| Solid—Liquid Extraction | Methanol and ethanol | Tannins | [53] | |
| Pressurized—Liquid Extraction | Ethanol | TPC (xanthones, phenolic acids, flavonoids, gallate derivatives and gallotannins) | [54] | |
| Microwave—Assisted Extraction | Ethanol | TPC (ethyl gallate, penta-O-galloyl-glucoside and rhamnetin-3-[6-2-butenoil-hexoside]) | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Mahecha, M.; Soto-Valdez, H.; Carvajal-Millan, E.; Madera-Santana, T.J.; Lomelí-Ramírez, M.G.; Colín-Chávez, C. Bioactive Compounds in Extracts from the Agro-Industrial Waste of Mango. Molecules 2023, 28, 458. https://doi.org/10.3390/molecules28010458
García-Mahecha M, Soto-Valdez H, Carvajal-Millan E, Madera-Santana TJ, Lomelí-Ramírez MG, Colín-Chávez C. Bioactive Compounds in Extracts from the Agro-Industrial Waste of Mango. Molecules. 2023; 28(1):458. https://doi.org/10.3390/molecules28010458
Chicago/Turabian StyleGarcía-Mahecha, Maribel, Herlinda Soto-Valdez, Elizabeth Carvajal-Millan, Tomás Jesús Madera-Santana, María Guadalupe Lomelí-Ramírez, and Citlali Colín-Chávez. 2023. "Bioactive Compounds in Extracts from the Agro-Industrial Waste of Mango" Molecules 28, no. 1: 458. https://doi.org/10.3390/molecules28010458
APA StyleGarcía-Mahecha, M., Soto-Valdez, H., Carvajal-Millan, E., Madera-Santana, T. J., Lomelí-Ramírez, M. G., & Colín-Chávez, C. (2023). Bioactive Compounds in Extracts from the Agro-Industrial Waste of Mango. Molecules, 28(1), 458. https://doi.org/10.3390/molecules28010458